Summary
In this study, we aimed to investigate the relationship between no-reflow phenomenon (NRP) and the systemic immune inflammation index (SII) (based on lymphocyte, neutrophil and platelet counts during percutaneous intervention) on saphenous vein grafts. A total of 133 patients who underwent percutaneous intervention for saphenous vein grafts due to acute coronary syndrome between 2019 and 2022 were included in this study. Our results showed that high SII levels were independently associated with the development of NRP in patients presenting with acute coronary syndrome and undergoing percutaneous intervention to the saphenous vein grafts.
Introduction
Coronary artery bypass grafting (CABG) is a revascularisation method used in selected patients with ischaemic heart disease [1]. Saphenous vein grafts (SVGs) are frequently used in CABG because they are easily accessible in daily clinical practice and do not have a significant effect on venous circulation in the lower extremities. However, 10–15% of SVGs become occluded in the first year after CABG surgery [2]. In the first decade, SVG patency rates are halved due to degenerative or occlusive disease [3]. Percutaneous interventions for occlusive lesions in SVGs are challenging and prone to complications. The condition called the no-reflow phenomenon, which describes impaired myocardial reperfusion after mechanical revascularisation of the infarct-related artery, is the leading complication. In patients presenting with acute coronary syndrome (ACS), the risk of no-reflow increases after the procedure is performed on these vessels if the culprit lesion is SVGs [4]. No-reflow phenomenon has multifactorial and complex pathogenesis, including distal embolisation, excessive thrombotic activity, microvascular spasm, inflammatory processes, the release of free oxygen radicals, and ischaemia–reperfusion injury [5]. No-reflow phenomenon is closely associated with adverse cardiovascular outcomes [6]. A predictive system for anticipating this phenomenon before the procedure could significantly enhance clinical efficacy by facilitating personalised treatment selection and empowering physicians to proactively implement preventive measures.
The systemic immune–inflammatory index (SII) is calculated based on neutrophil, lymphocyte, and platelet counts and is used as an indicator of the inflammatory status of the body [7]. The 3 inflammatory parameters that make up the SII are obtained from a complete blood count, and the calculation can be performed quickly. Numerous studies have shown that SII is a strong and independent prognostic indicator of the adverse outcomes of inflammatory diseases and malignant tumours [7–9]. Its high prognostic value has also been confirmed in various cardiovascular diseases, such as chronic heart failure and coronary artery disease [10–16]. However, its role in predicting the risk of no-reflow in percutaneous interventions to SVG has yet to be extensively investigated.
Aim
This study investigated the association between the SII and no-reflow phenomenon during percutaneous intervention in SVGs in CABG patients presenting with ACS. Our secondary objective was to evaluate the prognostic value of the SII in CABG patients presenting with ACS to assess death and major cardiac adverse events within 1 year after discharge.
Material and methods
Patient selection
The study included 314 patients with a history of previous CABG who were hospitalised between 2019 and 2022 with a diagnosis of ACS and who underwent percutaneous intervention of the SVG. Of these patients, 151 were excluded because the duration of the cine angiography images was not long enough to assess the myocardial blush grade (MBG) accurately. Thirty more patients with neoplastic diseases, a recent history of major surgery, active inflammatory or infective diseases, end-stage liver disease, and haematological disorders were excluded. The remaining 133 patients were retrospectively evaluated (Figure 1). The diagnosis of ACS was made by the current guidelines (Fourth Universal Definition of Myocardial Infarction [2018]) considering clinical symptoms, electrocardiographic changes, and changes in cardiac enzymes [17]. The study protocol was approved by the local Ethics Committee and complied with the Declaration of Helsinki.
Patient data
Demographic characteristics, cardiovascular risk factors, medications, and in-hospital mortality information were obtained from hospital records. After the index hospitalisation, routine 1-month, 6-month, and 1-year follow-up visits were performed, and information about possible recurrent infarction, hospitalisation, and mortality was recorded.
Complete blood count, creatinine, serum lipids, serum glucose, and C-reactive protein (CRP) were obtained from venous blood samples taken at the time of admission to the emergency department or initial hospitalisation in the coronary intensive care unit. The neutrophil-to-lymphocyte ratio (NLR) was calculated as the ratio of neutrophils to lymphocytes, and the platelet-to-lymphocyte ratio (PLR) was calculated as the ratio of platelets to lymphocytes. The SII was calculated as the ratio of the product of neutrophil count and platelet count to lymphocyte count [7]. Left ventricular ejection fraction (LVEF) was calculated using echocardiography performed within the first 24 h after hospitalisation using Simpson’s method.
Angiographic analysis and no-reflow phenomenon
All percutaneous intervention procedures were performed using a 6-Fr guiding catheter through the left radial or right femoral route using standard techniques. All patients were treated with 300 mg of aspirin and 180 mg of ticagrelor, clopidogrel at an appropriate dose (300 mg or 600 mg), or 60 mg of prasugrel in appropriate patients before the procedure. During the procedure, 50–70 U/kg of unfractionated heparin was administered. The decision to use mechanical thrombectomy, intracoronary glycoprotein IIb/IIIa receptor blockers, adrenaline, adenosine, or nitroglycerin was left to the operator. Coronary angiography recordings were obtained using a Toshiba Infinix 8000 V and Toshiba Infinix 8000 G5 (Toshiba Medical Systems, Nasushiobara, Japan). Two blinded interventional cardiologists analysed the angiographic images, and if necessary, a third blinded interventional cardiologist was consulted. Angiographic thrombus grade was visually assessed as follows: grade 0: no angiographic features of the thrombus; grade 1: angiographic features of the possible thrombus (reduced contrast intensity, blurring, irregular lesion contour, or a smooth convex “meniscus” at the site of occlusion); grade 2: definite thrombus with the largest dimension less than or equal to half the vessel diameter; grade 3: definite thrombus with the largest dimension greater than half but less than or equal to twice the vessel diameter; grade 4: definite thrombus with the largest dimension greater than twice the vessel diameter; and grade 5: total vessel occlusion due to thrombus [18].
No-reflow phenomenon is defined as inadequate myocardial tissue perfusion after a transient period of ischaemia without signs of mechanical obstruction such as dissection, spasm, or thrombus in the epicardial artery [19]. The no-reflow phenomenon concept also encompasses the phenomenon of slow flow [19]. The TIMI angiographic scale using grades 0, 1, 2, and 3 before and after percutaneous intervention was used to determine epicardial blood flow patterns [20]. Post-procedural myocardial perfusion was evaluated using the MBG method based on the procedure described by van’t Hof et al. [21]. Through this method, patients with no contrast intensity were graded as MBG 0, patients with minimal contrast intensity as MBG 1, patients with moderate but below normal contrast intensity as MBG 2, and patients with normal contrast intensity as MBG 3. Angiographically, no-reflow was defined as a post-procedural TIMI flow grade score ≤ 2 or MBG < 2 and a TIMI flow grade score of 3 [22].
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 21.0; SPSS Inc., Chicago, Illinois, USA). The continuous data were expressed as the mean ± standard deviation or median (interquartile range) depending on normality, assessed using the Kolmogorov-Smirnov test. The categorical variables were expressed as counts (n) and percentages (%). According to normality, the group means for continuous variables were compared using either the independent samples t-test or the Mann-Whitney U test. Spearman’s correlation coefficients were used to determine the correlations between the SII and the no-reflow.
As appropriate, the categorical variables were compared using the χ2 test or Fisher’s exact test. The association of different variables with the no-reflow phenomenon and 1-year mortality was calculated in univariate analysis. Variables showing marginal associations with the no-reflow and 1-year mortality on univariate testing (p < 0.50) were included in the multivariate regression analysis. The stepwise method with backward elimination was used. Odds ratios with 95% confidence intervals were also calculated. Receiver operating characteristic (ROC) curve analysis was performed to define thresholds for SII for predicting the no-reflow with corresponding specificity and sensitivity. A two-sided p < 0.05 was considered statistically significant. ROC curve analysis was performed to define the thresholds for the SII for predicting the no-reflow with the corresponding specificity and sensitivity.
Results
Of the 133 patients included in the study, 115 were male and 18 were female. The demographic, clinical, and laboratory characteristics of the patients are summarised in Table I. Forty-seven of the patients were hospitalised due to ST-elevation myocardial infarction (STEMI), and the remaining 86 were hospitalised due to non-ST-elevation myocardial infarction (NSTEMI). Reperfusion was achieved in 84 (63%) patients, and no-reflow developed in 49 (37%) patients. The white blood cells (WBC) and neutrophil values were significantly higher in the no-reflow group (8800 [7000, 10,900] vs. 9900 [8200–12,400], p = 0.048; 5900 [4400, 7600] vs. 7100 [5700, 9500]; p = 0.002), while the lymphocyte values were significantly lower in the no-reflow group (2200 [1700, 2700] vs. 1900 [1300, 2300], p = 0.043). The median value of SII was significantly higher in patients with no-reflow in comparison with normal reperfusion (543 [447, 717] vs. 861 [642, 1272], p < 0.001). The NLRs and PLRs were also significantly higher in patients with no-reflow (2.68 [2.09, 3.57] vs. 3.90 [2.96, 5.75]; p < 0.001, 92.44 [75.81, 119.69] vs. 129.46 [99.47, 156.96], p < 0.001).
Table I
Baseline demographic and clinical characteristics
| Characteristics | Normal reperfusion n = 84 | No-reflow n = 49 | P-value |
|---|---|---|---|
| Age [years] | 68 (62, 76) | 64 (55.5, 71) | 0.03 |
| Gender (male), n (%) | 72 (73) | 43 (88) | 0.80 |
| Smoking, n (%) | 36 (43) | 22 (42) | 0.37 |
| Hypertension, n (%) | 56 (67) | 28 (57) | 0.35 |
| Diabetes mellitus, n (%) | 37 (44) | 23 (47) | 0.86 |
| Prior stroke, n (%) | 3 (4) | 1 (2) | 1.00 |
| Haemoglobin [mg/dl]* | 13.2 ±2.1 | 13.4 ±2 | 0.71 |
| WBC count [µl]* | 8800 (7000, 10900) | 9900 (8200, 12400) | 0.05 |
| Platelet count [× 109/l]* | 198 (171, 244) | 225 (171, 268.5) | 0.11 |
| Neutrophil count [µl]* | 5900 (4400, 7600) | 7100 (5700, 9500) | 0.002 |
| Lymphocyte count [µl]* | 2200 (1700, 2700) | 1900 (1300, 2300) | 0.04 |
| Creatinine [mg/dl]* | 1 (0.8, 1.3) | 1 (0.8, 1.3) | 0.99 |
| Total cholesterol [mg/dl]* | 192 ±58.8 | 194.3 ±60.7 | 0.89 |
| Triglyceride [mg/dl]* | 113 (84, 180.8) | 120 (70, 178.5) | 0.70 |
| HDL-C [mg/dl]* | 41 (36, 47.8) | 40 (33.5, 49) | 0.60 |
| LDL-C [mg/dl]* | 136.3 ±46.4 | 138.7 ±49.3 | 0.68 |
| Glucose [mg/dl]* | 149.5 (111.3, 227.3) | 157 (112.5, 236) | 0.44 |
| Hs-CRP [mg/dl]* | 4.8 (3.1, 11.9) | 4.5 (3.2, 13.1) | 0.76 |
| Neutrophil/lymphocyte ratio* | 2.7 (2.1, 3.6) | 3.9 (3, 5.8) | < 0.001 |
| Platelet/lymphocyte ratio* | 92.4 (75.8, 119.7) | 129.5 (99.5, 157) | < 0.001 |
| Systemic immune inflammation index* | 543 (447, 717) | 861 (642, 1272) | < 0.001 |
| NonSTEMI, n (%) | 57 (68) | 28 (57) | 0.26 |
| Killip > 2, n (%) | 6 (7) | 8 (16) | 0.14 |
| LVEF (%) | 45 (40, 50) | 45 (32.5, 50) | 0.42 |
| In-hospital mortality, n (%) | 3 (4) | 5 (10) | 0.14 |
| One-year mortality, n (%) | 7 (8) | 13 (27) | 0.01 |
| Medications in hospital, n (%) | |||
| Dual antiplatelet treatment, n (%) | 84 (100) | 49 (100) | NS |
| ACEI, n (%) | 66 (79) | 35 (71) | 0.40 |
| ARB, n (%) | 3 (4) | 1 (2) | 1.00 |
| β-blocker, n (%) | 72 (86) | 41 (84) | 0.80 |
| Statin, n (%) | 82 (98) | 46 (94) | 0.36 |
* IQR: interquartile range; WBC – white blood cell, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, hs-CRP – high-sensitivity C-reactive protein, nonSTEMI – non-ST elevation myocardial infarction, LVEF – left ventricular ejection fraction, ACEI – angiotensin-converting enzyme inhibitor, ARB – angiotensin II receptor blocker.
Moreover, there was no significant difference between the groups with and without no-reflow in terms of LVEF and Killip > 2 at index hospitalisation (45 [40, 50] vs. 45 [32.5, 50], p = 0.422; 7% vs. 16%, p = 0.14). While there was no statistically significant difference in in-hospital mortality between the no-reflow and normal perfusion groups at index hospitalisation (4% vs. 10%, p = 0.14), the 1-year mortality rate was significantly higher in the no-reflow group (8% vs. 27%, p = 0.01).
The angiographic characteristics of the study population are summarised in Table II. There was no significant difference in no-reflow in cases in which the culprit lesion affected the inferior region of the heart (19% vs. 35%, p = 0.06). No statistically significant difference was found in pre-dilatation, post-dilatation, stent diameter, and size between the normal perfusion and no-reflow groups. Thrombus aspiration and glycoprotein IIb–IIIa inhibitor use were significantly higher in the no-reflow group (5% vs. 18%, p = 0.02, 21% vs. 51%, p < 0.001).
Table II
Angiographic and procedural characteristics
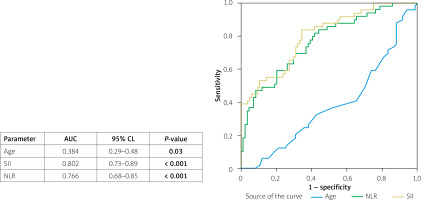
ROC curve analysis further showed that SII was a prominent predictor of the no-reflow phenomenon with an AUC of 0.80 (95% CI: 0.73–0.89, p < 0.001) as compared with other predictors of no-reflow (NLR; AUC = 0.76 (95% CI: 0.68–0.85, p < 0.001) and age; AUC, 0.38 (95% CI: 0.29-0.48, p = 0.03). The optimal threshold for SII in predicting the no-reflow phenomenon was 613, with sensitivity and specificity of 84% and 66%, respectively (Figure 2).
Figure 2
ROC curve analysis for age, systemic immune-inflammation index (SII), and neutrophil to lymphocyte ratio (NLR)
The parameters that could be risk factors for no-reflow in ACS patients undergoing saphenous intervention were evaluated using logistic regression analysis (Table III). For this purpose, age, diabetes mellitus, inferior infarct location, Killip > 2, NLR, PLR, SII ≥ 613, total stent length, initial TIMI score > 2, and thrombus grade ≥ 3 were analysed in the context of whether they were correlated with no-reflow. Age, NLR, PLR, SII ≥ 613, total stent length, and thrombus grade ≥ 3, which were risk factors found to be correlated with no-reflow as a result of the univariate logistic regression analysis, were also analysed using multivariate logistic regression analysis. Accordingly, age, NLR, SII ≥ 613 (odds ratio: 4.02, 95% CI = 1.40–11.57, p < 0.01), and thrombus grade ≥ 3 were found to be independent predictors of no-reflow in percutaneous intervention of SVG in patients with ACS.
Table III
Significant predictors of no-reflow in univariate and multivariate logistic regression analyses
The associations between different variables and 1-year mortality after the procedure were evaluated using univariate analysis. Variables with a p < 0.5 were analysed using a multivariate logistic regression model in univariate analysis. According to the multivariate regression analysis, age, Killip > 2, and NLR values were independent predictors of 1-year mortality (Table IV).
Table IV
Effects of various variables in 1-year mortality in univariate and multivariate logistic regression analyses
Discussion
In our study, we mainly investigated the relationship between the SII and no-reflow phenomenon, which is one of the most important complications of percutaneous intervention of SVGs in patients presenting with ACS. According to the results of our study, SII was found to be an independent predictor of no-reflow phenomenon in patients presenting with ACS and undergoing percutaneous intervention of the SVG.
Depending on the methods used to assess no-reflow phenomenon, studies have shown that it increases by 5%–50% after primary percutaneous coronary intervention (PCI) [23]. Both ACS and SVG interventions are risk factors for the development of no-reflow [24]. In Gürbak et al.’s study, the rate of no-reflow development was 29% in interventions performed in the SVG [25]. In another study, this rate was 18% in patients undergoing PCI of the SVG [26]. Özen et al. found a no-reflow rate of 31% in 124 patients who presented with ACS and who underwent PCI of the SVG [27]. However, in this study, angiographic no-reflow was defined as TIMI flow grade < 3. Patients with TIMI flow grade > 3 and MBG < 2 were not included in the definition of no-reflow. In our research, the no-reflow rate was approximately 37%. The reason for the higher rate in our study compared with the literature may be that we used a more sensitive method to evaluate no-reflow phenomenon.
Compared with atherosclerotic plaques in native coronary arteries, atherosclerotic plaques in SVGs are more diffuse and fragile and have less calcification. In addition, plaques in SVGs contain more inflammatory cells and fewer fibrous caps. Because of these different characteristics, SVG lesions are more prone to distal embolisation, resulting in angiographic no-reflow and distal microvascular occlusion [28]. To prevent such complications, the use of emboli protection devices is recommended for procedures conducted on SVGs [29]. However, in contemporary PCI, emboli protection devices are used in only 14–21% of patients [29]. In some clinical studies, distal protection devices have been shown to increase the no-reflow rate in procedures performed on SVGs [28]. This is thought to be due to the use of emboli protection devices in older patients with a higher thrombus burden [28]. Emboli protection devices were not used in the patient population in our study, which contributed to its high no-reflow rate.
Previous studies have shown that molecular interactions between leukocytes and platelets trigger no-reflow phenomenon by increasing local inflammatory activity at the microcirculatory level [23, 30]. Neutrophilia has also been shown to be highly associated with no-reflow in patients undergoing PCI for ACS [31]. Activated neutrophils adhere to the endothelium in capillaries and become trapped in microvessels, thus contributing to changes in endothelial cells and vessel occlusion. Furthermore, activated neutrophils release a variety of inflammatory mediators, including various cytokines (e.g. tumour necrosis factor-α and interleukin-1β), free oxygen radicals, and proteolytic enzymes that directly cause microvascular endothelial damage. At the same time, neutrophils combine with platelets to form neutrophil–platelet aggregates that inhibit microcirculation, thus mechanically arresting blood flow. Activated platelets release inflammatory mediators into the microcirculation, causing the activation of other platelets and promoting the retention of more platelets, thus creating a vicious cycle of inflammation and coagulation [32]. In addition, a prothrombotic state resulting from thrombocytosis may contribute to the development of no-reflow [33]. Lymphocytopaenia has been reported to be significantly associated with the progression of atherosclerosis and major adverse cardiac outcomes in patients with acute myocardial infarction [34]. Several possible causes of lymphopaenia in ACS patients have been proposed, including sudden increased corticosteroid levels, neurohormonal hormone levels, and increased lymphocyte apoptosis due to uncontrolled immune system activation [35, 36]. In conclusion, a decreased lymphocyte count indicates severe inflammation and may lead to an increased risk of no-reflow.
Due to the effects of inflammatory cells on the pathophysiology of no-reflow phenomenon, there has been increasing interest in the clinical use of various inflammatory ratios to predict the development of no-reflow. Several studies have suggested that it can predict the risk of NLR and PLR. Sen et al. found that a high NLR was associated with both no-reflow phenomenon and long-term prognosis in STEMI patients undergoing primary PCI [37]. Vatan et al. found a high NLR to be one of the independent predictors of no-reflow in STEMI patients [15]. Toprak et al. showed that the PLR value at admission was an independent predictor of no-reflow phenomenon in STEMI patients [38]. Kocas et al. showed that the NLR was an independent predictor of a high TIMI flow grade [39]. In another study, Vakili et al. found that both PLR and NLR correlated with the TIMI flow grade and were effective indices for predicting no-reflow phenomenon in STEMI patients undergoing primary PCI [40]. However, these models (i.e. NLR and PLR) only indicate local immune and inflammatory status, whereas inflammation is often systemic.
SII is a value derived from neutrophil, platelet, and lymphocyte counts that can reflect comprehensive immune and inflammatory status. Initially used to predict clinical outcomes in inflammatory diseases and malignant tumours, the SII has proven to be a promising prognostic marker in various cardiovascular diseases. Selçuk et al. found that SII was an independent predictor of postoperative atrial fibrillation in patients undergoing isolated CABG [13]. Vatan et al. showed that the SII was an independent predictor of the development of no-reflow phenomenon in patients who presented with STEMI and underwent primary PCI [15]. Recently, Özen et al. found that SII was an independent predictor of the development of no-reflow in patients who presented with ACS and underwent percutaneous intervention of SVGs [27]. However, they used the TIMI flow grade method to define the no-reflow phenomenon. A TIMI flow grade between 0 and 2 is predictably associated with no-reflow phenomenon. However, the sensitivity of the TIMI flow grade is relatively low. The no-reflow phenomenon can also occur after successful large epicardial vessel recanalisation, resulting in TIMI flow grade 3. MBG is a more sensitive method for assessing no-reflow phenomenon after percutaneous intervention [41]. MBG is a semiquantitative method that shows myocardial tissue perfusion after contrast agent injection. In our study, a combination of the TIMI flow grade and MBG was used to assess no-reflow phenomenon.
In our study, we demonstrated that age, NLR, SII ≥ 613, and thrombus grade ≥ 3 score were strong independent predictors of the development of no-reflow in patients with ACS undergoing SVG procedures. When SII, age, and NLR were compared in the ROC curve analysis, SII had the most significant predictive value for no-reflow. This result is consistent with a previous report [27].
No-reflow phenomenon is an important complication that is more common in patients undergoing CABG surgery and has negative impacts on mortality and morbidity. Therefore, an easy and feasible method that can predict the development of no-reflow before the procedure is important in terms of possible complications to be encountered during the intervention. The relationship between inflammation and the extent of atherosclerosis has been studied for many years. SII is a fast and easily obtained value that can be calculated with inflammatory parameters using a complete blood count. This correlation between SII and no-reflow might help operators to be prepared for possible complications.
Recently, studies have shown that SII can predict prognoses in ACS patients. Huang et al. found that SII was an independent predictor of in-hospital mortality and long-term mortality in elderly patients with acute myocardial infarction undergoing percutaneous intervention [42]. Vatan et al. showed that SII was independently correlated with 30-day mortality in STEMI patients [15]. Conversely, Gur et al. found no relationship between SII and mortality in ACS patients [43]. In our study, we found no association between 1-year mortality and SII.
This study has some limitations. First, this study has a single-centre, retrospective, and cross-sectional design. Again, this study was performed using a relatively limited patient group. Second, we conducted this study using only the entry levels of SII. The SII levels after the acute phase of ACS were not assessed, which restricted the study’s relevance, particularly concerning long-term outcomes such as mortality. Third, complex imaging modalities, such as myocardial contrast echocardiography and cardiac magnetic resonance imaging, were not used for the diagnosis of no-reflow phenomenon. Therefore, prospective studies with larger populations are needed to confirm our results.
Conclusions
This study showed that high SII levels, which could be easily calculated with a single complete blood count test, were independently associated with the development of no-reflow phenomenon in patients who presented with ACS and who underwent percutaneous intervention of the SVG. However, no association was found between 1-year mortality and SII levels in the same patient group.