Introduction
Inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), are chronic conditions characterised by immune dysregulation, genetic predisposition, impaired intestinal barrier function, and alterations in gut microbiota. A key feature of these disorders is the central role of macrophages in mediating inflammation, primarily through the production of pro-inflammatory cytokines (e.g. TNF-α, IL-6, IL-8), nitric oxide (NO), and reactive oxygen species, contributing to mitochondrial dysfunction. Current therapeutic strategies for IBD include immunomodulators, aminosalicylates, corticosteroids, and biologic agents. Recent research, however, indicates that hyperbaric oxygen therapy (HBOT) may offer a novel anti-inflammatory approach by modulating cytokine production. Notably, HBOT has demonstrated greater clinical efficacy in CD compared to UC [1, 2]. Under normal physiological conditions, oxygen transport is predominantly haemoglobin-mediated, achieving approximately 98% saturation, with a minor fraction dissolved in plasma. In contrast, HBOT significantly increases plasma-dissolved oxygen levels due to elevated partial pressures of inhaled oxygen [3].
HBOT is a therapeutic modality extensively used for treating various conditions, including recalcitrant wounds, radiation-induced inflammation, and carbon monoxide poisoning. The therapeutic efficacy of HBOT primarily stems from a significant increase in reactive oxygen species (ROS) levels, which promotes the synthesis of key growth factors such as vascular endothelial growth factor (VEGF), placental growth factor (PGF), and angiopoietins (Ang) 1 and 2. Additionally, HBOT enhances the mobilisation of bone marrow-derived stem cells, crucial for neovascularisation and tissue repair and regeneration. An intriguing aspect of HBOT is the induction of hyperoxia, which modulates the release of nuclear factor κB (NF-κB), reducing the transcription of pro-inflammatory cytokine genes. This response fosters an anti-inflammatory state despite the presence of oxidative stress [4, 5]. This complex molecular interplay highlights the therapeutic potential of HBOT in promoting tissue healing and reducing inflammation across various clinical scenarios.
CD, a chronic inflammatory disorder of the gastrointestinal tract, presents significant challenges due to its focal or segmental transmural inflammation, affecting different parts of the digestive system. This inflammation pattern compromises intestinal mucosal integrity, predisposing to abscesses and fistula formation. Notably, fistulas in CD can be internal, involving adjacent organs, or external, connecting to the skin surface, with perianal fistulas being a significant subtype. These represent abnormal connections between the anal canal’s epithelialised surface and the surrounding skin. Research indicates that approximately 20% to 25% of individuals with CD develop perianal fistulas [6–8]. Clinically, perianal fistulas manifest with distressing symptoms such as anal pain, purulent discharge, skin irritation, and perianal swelling, severely impacting patients’ quality of life. This underscores the importance of effective management strategies for this complex manifestation of CD.
Managing perianal fistulas in CD requires a comprehensive, interdisciplinary approach. The initial step is a meticulous diagnosis, involving thorough examination of the anal and perianal regions.
Aim
This study investigates the impact of HBOT on perianal fistula activity in a cohort of 11 patients with CD, each presenting with perianal fistulas.
Material and methods
Study design and participants
A cohort of 16 patients diagnosed with CD, under the care of the Gastroenterology Department at the Ministry of the Interior and Administration Hospital in Gdansk, was selected for this study. To ensure the study’s rigor and relevance, stringent inclusion criteria were established. These criteria required patients to have both active CD and active perianal fistulas, with a minimum disease duration of 3 years. Patients’ treatment histories were meticulously documented during interviews. This documentation included conventional treatments such as mesalazine, periodic steroids, and immunosuppressive drugs (azathioprine, 6-mercaptopurine, methotrexate). Additionally, records of surgical interventions for perianal fistulas and participation in biological treatment regimens, in line with the current drug programme, were included. A crucial aspect of the cohort’s profile was the history of frequent disease recurrences and persistent, variable levels of fistula activity.
To ensure methodological rigor and specificity of results, the study excluded individuals based on the following criteria: a disease duration of less than 3 years, inactive perianal fistulas, presence of rectovaginal or interintestinal fistulas, anal stenosis as a disease manifestation, comorbid cardiovascular conditions, cancer, claustrophobia, or lack of consent for participation. Patients were enrolled in a carefully designed treatment program, with efficacy assessments conducted at various stages. The regimen included conventional treatments with immunosuppressants such as azathioprine, 6-mercaptopurine, and methotrexate. Additionally, selected patients received biological drugs as supplementary therapy. Alongside these standard treatments, all participants underwent concurrent HBOT. Prior to initiating HBOT, each patient underwent surgical intervention by the same experienced proctologist (A.B.). The surgical approach was customised to each patient’s specific condition. For simple fistulas, fistulotomy was performed. In cases of complex fistulas, a staged surgical approach was adopted. The initial stage involved a comprehensive procedure using the TROPIS method, which entailed the meticulous opening of all fistulas and potential abscesses, including those in the intersphincteric space.
The management of transsphincteric fistulas involved systematic drainage using a silicone seton until significant remission of local inflammation was achieved. Following this phase, a joint evaluation by a gastroenterologist and a surgeon was conducted, utilising endoscopic, magnetic resonance imaging (MRI), and transrectal ultrasound (TRUS) assessments. This comprehensive evaluation informed the selection of the next surgical step. In cases where the mucosa around the internal orifice showed resolution or minimal inflammatory changes, closure was achieved using either a displaced flap technique or the ligation of intersphincteric fistula tract (LIFT) procedure. Conversely, if persistent inflammation or ulceration was present around the internal orifice, a seton was maintained, traversing the fistula’s primary canal. Treatment strategies also focused on lateral canals, often combining fistulotomy with marsupialisation.
Disease activity was evaluated using the Crohn’s Disease Activity Index (CDAI) [9] along with relevant laboratory tests, including stool calprotectin, C-reactive protein (CRP) activity, serum iron levels, and peripheral blood haemoglobin values. Endoscopic activity was assessed using the Simple Endoscopic Score for Crohn’s Disease (SES-CD) [10], while the Perianal Crohn’s Disease Activity Index (PDAI) [11, 12] was used for MRI fistula activity assessment. These measures provided a comprehensive evaluation of the disease’s clinical status. Despite the small sample size, the study also aimed to assess the impact of hyperbaric treatment on patients concurrently receiving biological drugs, treating the results as indicative trends.
Eligible patients underwent a rigorous qualification process for hyperbaric treatment, overseen by a hyperbaric medicine specialist. Contraindications such as pregnancy, pulmonary bullae, and severe heart insufficiency were carefully considered. The HBOT sessions were conducted at the Department of Hyperbaric Medicine and Sea Rescue, University Centre for Maritime and Tropical Medicine in Gdynia, affiliated with the Medical University of Gdansk, Poland. The standard protocol for non-healing wounds was applied.
HBOT sessions occurred 5 times a week (Monday to Friday), totalling up to 30 sessions. Treatments were administered in a multiplace hyperbaric chamber, under the supervision of qualified medical personnel. Each session lasted approximately 90 min, including compression, 60 min of exposure to 100% oxygen at 2.5 ATA, and decompression. A comprehensive assessment scheme was employed to systematically evaluate and observe the therapeutic outcomes of the treatment. The treatment effects were assessed in accordance with the following scheme:
Stage I – preliminary
Colonoscopy with SES-CD activity scores.
Contrast-enhanced MRI of the small pelvis with the assessment of fistula activity.
Chest X-ray.
ECG.
ECHO of the heart in patients suffering from cardiological conditions.
Laboratory tests: complete blood count, iron levels, CRP, and calprotectin in the stool.
Assessment of disease activity – CDAI.
Consultation with a proctologist.
Consultation of a hyperbaric medicine doctor.
Stage V – after 9 months, follow-up
Assessment of activity on the CDAI scale.
Laboratory tests: complete blood count, level of Fe, CRP, calprotectin in the stool.
Colonoscopy with the assessment of activity on the SES-CD scale.
Contrast-enhanced pelvic MRI with the assessment of fistula activity.
Consultation with a proctologist.
Eleven patients were further analysed. Of these, 5 were concurrently treated with biological drugs: 3 received infliximab and 2 received adalimumab. Biological treatment was initiated after fistula preparation and prior to hyperbaric therapy. All patients in the group received immunosuppressive treatment, and their perianal fistulas were addressed surgically.
Hypotheses
Considering the study’s design, we categorised the hypotheses into primary and secondary outcomes, based on the predicted results. Consequently, the following hypotheses have been formulated:
Primary hypotheses:
H0: There is no significant difference in the CDAI results at all time points pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H1: There are significant differences in the CDAI results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H0: There is no significant difference in the SES-CD results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H2: There are significant differences in the SES-CD results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H0: There is no significant difference in the PDAI results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H3: There are significant differences in the PDAI results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
Secondary hypotheses:
H0: There is no significant difference in the calprotectin results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H4: There are significant differences in the calprotectin results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H0: There is no significant difference in the Fe results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H5: There are significant differences in the Fe results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H0: There is no significant difference in the haemoglobin (Hb) results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H6: There are significant differences in the Hb results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H0: There is no significant difference in the CRP results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
H7: There are significant differences in the CRP results at all time points (pre-treatment and in stages III, IV, and V) before and after the HBOT treatment.
Statistical analysis
We analysed data using SPSS software, version 27.0. Descriptive statistics, including mean, median, and standard deviation, were employed for data characterisation. For the comparison of normally distributed parametric data, we utilised one-way analysis of variance (ANOVA) with repeated measures, applying the Bonferroni correction. The Friedman test was conducted to analyse non-normally distributed data. Furthermore, for each group combination, we performed post-hoc Wilcoxon tests with Bonferroni corrections. The threshold for statistical significance was set at p < 0.05.
Results
A total of 11 patients, comprising 3 women and 8 men, participated in this study. However, 2 patients experienced side effects from hyperbaric treatment – one with hearing impairment and another with headaches. Additionally, 3 patients were excluded due to incomplete examinations because they failed to attend scheduled check-ups. The average age of the participants was 30.6 years. We evaluated the effectiveness of HBOT by analysing various parameters: CRP, CDAI, SES-CD, calprotectin, Fe, Hb, and fistula activity. Table I presents the mean and standard deviation of these dependent parameters at different stages of the study.
Table I
Descriptive statistics for CRP, CDAI, SES-CD, calprotectin, Fe, Hb, and fistula activity
Three of the variables were normally distributed (SES-CD, Fe, and fistula activity). Thus, we applied the one-way ANOVA with repeated measures.
Table II shows the results of Mauchly’s test of sphericity, and Table III shows the results of a test of within-subject effects. Mauchly’s test, also known as Mauchly’s sphericity test, is a statistical test used in the context of analysis of variance to assess the assumption of sphericity. Sphericity is an assumption in ANOVA that is necessary for valid results when conducting repeated measures ANOVA. In particular, it refers to the idea that the variances of the differences between all possible pairs of related groups are equal. Violating this assumption can lead to a higher probability of incorrectly rejecting a null hypothesis and, thus, can affect the overall accuracy of results. In case Mauchly’s test indicated a violation of sphericity (Table II), the Greenhouse-Geisser correction, that adjust the degrees of freedom in the ANOVA to account for the violation of sphericity, was applied (Table III).
Table II
Results of Mauchly’s test of sphericity
Table III
Test of within-subject effects for each of the parameters (variables) (normally distributed): one-way ANOVA with repeated measures
For SES-CD, the p-value from Mauchly’s test was 0.060 indicating no violation of the sphericity assumption, thus the univariate repeated measures ANOVA without the G-G correction was applied and indicated that there were significant differences in SES-CD scores over time (p < 0.001) (Table III). The partial eta-squared indicates a large effect size (0.723). The post hoc paired comparisons for SES-CD indicated that there were significant differences between stage I (pre-treatment) and stage IV as well as between stages I and V. However, there were not statistically significant differences between stages IV and V (Table IV).
Table IV
Results of post hoc comparisons for parameters normally distributed: one-way ANOVA with repeated measures
As regards fistula activity, the p-value from Mauchly’s test was 0.286, again, indicating no violation of sphericity assumption and thus suggesting that the use of the G-G test is unnecessary. The univariate repeated measures ANOVA using the G-G correction indicated there were significant differences in fistula activity scores over time (p < 0.001) (Table III). The partial eta-squared indicates a large effect size (0.550). The post hoc paired comparisons for fistula activity indicated that there were significant differences between stage I (pre-treatment) and stage IV and V (Table IV).
As for Fe, the Mauchly test reached the value of 0.066 with the p-value < 0.001, indicating the violation of the sphericity assumption. Additionally, the Greenhouse-Geisser correction was below 0.75, which suggest that the G-G correction should be used to assess the ANOVA results. The univariate repeated measures ANOVA with G-G correction indicated that there were no significant differences in Fe scores over time (p = 0.477) (Table III). Thus, we did not conduct the post hoc paired comparisons for Fe.
Four of the variables were non-normally distributed (CRP, CDAI, calprotectin, and Hb). Thus, we applied the Friedman test, which is a non-parametric statistical test similar to the ANOVA with repeated measures (Table V). For CRP, CDAI, and calprotectin there were significant differences over time (respectively, p = 0.021, p = 0.048, and p = 0.006). For the Friedman test, Kendall W test was used as a measure of the effect size. Furthermore, for CRP, CDAI, and calprotectin, post-hoc comparisons were conducted using the Wilcoxon signed rank test, indicating a moderate effect for calprotectin and small effects for CRP and CDAI.
Table V
Test of within-subject effects for each of the parameters (variables) (non-normally distributed): Friedman test
To recapitulate, all the hypotheses, with the exception of H5 and H6, were supported. Table VI presents a summary of the effects of HBOT on CD activity.
Table VI
Effect of treatment on Crohn’s disease activity
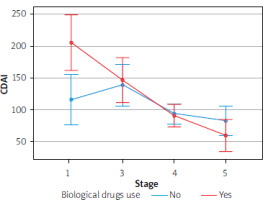
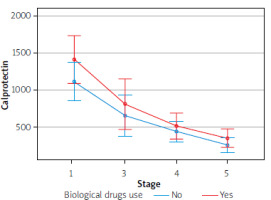
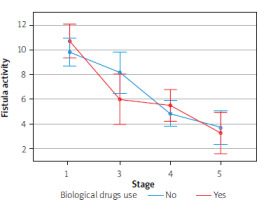
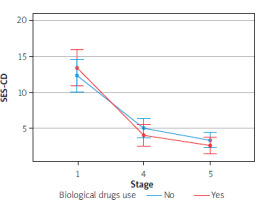
Furthermore, there was no effect of biological drugs on the results of the parameters in question, due to a small group sizes. However, we detected a tendency to enhance the therapeutic effect with simultaneous use of biological treatment, based on such parameters as CDAI, stool calprotectin level, endoscopic activity of the disease, or fistula activity
The Figures 1–4 illustrate the influence of HBOT on the activity of perianal fistulas in patients with or without biological treatment. This analysis includes both patients treated with biological drugs and those who did not receive such treatment. As previously noted, this involved a small cohort; therefore, the findings should be interpreted as indicative trends rather than definitive conclusions.
Discussion
HBOT, a pioneering approach in wound healing, involves the therapeutic administration of 100% oxygen at increased atmospheric pressures, typically between 2 to 3 atm. This advanced therapeutic method utilises oxidative stress to accelerate and enhance healing in various medical conditions.
The hyperoxidation process induces a significant increase in the production of free oxygen radicals (ROS), along with elevated levels of lactate and nitric oxide at the injury site. This orchestrated biochemical cascade promotes cellular recruitment and increased protein synthesis, thereby facilitating wound healing [13]. To address the complex challenge of perianal fistulas, particularly those resistant to healing, we employed HBOT in our patient cohort. In this study, 11 patients with a history of perianal fistulas and varying degrees of CD severity, as assessed by the CDAI, were deemed eligible. Notably, all patients had active perianal fistulas, making them suitable for our comprehensive treatment program.
After surgical intervention for fistulas, some patients received adjunctive biological treatment (anti-TNF-α) alongside conventional therapy. This strategy aimed not only to accelerate the healing of perianal fistulas but also to manage enteritis, potentially leading to clinical improvement as reflected by the CDAI, and ultimately achieving clinical remission. The effectiveness of this combined treatment approach was evaluated based on biochemical parameters, endoscopic disease activity, and the regression of perianal lesions as assessed by MRI. A systematic review exploring the optimal number of HBOT sessions for therapeutic efficacy highlighted the beneficial effects of combining HBOT with standard management in patients with IBD, including CD and UC. Interestingly, no significant difference in therapeutic outcomes was observed between 20 and 40 HBOT sessions. It was also found that a 5-day oxygen supply regimen yielded better results compared to a 3-day regimen, positively influencing disease activity, bleeding intensity, recurrence rates, and subclinical inflammation. Moreover, in CD, this approach was found to affect the risk of developing strictures and fistulas [14].
The treatment regimen was meticulously established, involving a frequency of 5 days per week over a 6-week period, culminating in a total of 30 HBOT sessions. The rationale for this comprehensive approach was twofold: firstly, to evaluate the clinical efficacy of the treatment, with assessments conducted in the sixth week following HBOT completion; and secondly, to investigate any potential long-term effects on disease recurrence, with follow-up assessments of disease activity scheduled approximately 9 months post-treatment. This carefully designed protocol aimed to assess not only the immediate effects of treatment but also to monitor any sustained benefits over an extended period post-treatment. A similar protocol has been employed in other studies investigating HBOT in IBD [15]. The consistent use of this regimen across various research studies enhances the ability to conduct a robust and comparative evaluation of the therapeutic benefits of HBOT in IBD cases.
Numerous studies have consistently shown that HBOT is a safe and effective treatment for various phenotypes and complications associated with IBD, including perianal fistulas, J-pouchitis, and skin complications like pyoderma gangrenosum. A meta-analysis incorporating 3 randomised trials and 16 case series reported promising outcomes, with high rates of clinical remission in UC and CD, achieving an impressive 87–88%. Although the remission rate for treating perianal fistulas in CD was relatively low, it still reached a considerable 48–60% [16, 17]. HBOT has also been recognised as a beneficial adjunctive treatment in severe flares of UC and the fistula form of CD [18]. In line with these findings, our study also yielded noteworthy results. The clinical effect, assessed using the CDAI, reached a substantial level of 81.8% approximately 9 months post-HBOT treatment. Regarding calprotectin levels, with a cut-off point of 250 µg/g, the clinical effect was a commendable 72.8%. Significantly, we observed substantial healing of intestinal mucosal lesions, as evaluated by the SES-CD, in 54.5% of patients. Additionally, a parallel response in fistula healing was noted, assessed using the PDAI, with 54.5% of patients achieving 0–2 points on the PDAI scale, indicative of fistula healing and coinciding with endoscopic remission. Importantly, these encouraging results were not only evident after the initial 6-week post-HBOT assessment but also sustained over the longer follow-up period.
The beneficial impact of HBOT on reducing the frequency of relapses in IBD may be attributed to its role in downregulating the expression of hypoxia-inducible factor-1α (HIF-1α). Our study produced noteworthy findings, demonstrating that patients undergoing HBOT showed reduced expression of inflammatory markers such as TNF-α, IL-6, vascular endothelial growth factor, and HIF-1α. These results suggest that HBOT not only suppresses inflammatory processes and alleviates hypoxia in tissues but also potentially enhances immune responses, leading to decreased disease recurrence. The mechanism involving HIF-1α, which binds to the promoter of the TNF-α gene and increases its expression, underscores a link between HIF-1α and inflammation. Reducing HIF-1α expression seems to diminish inflammation and facilitate mucosal healing [19, 20]. This therapeutic mechanism is reflected in our study, where we observed a statistically significant decrease in calprotectin levels both at the 12th week (6 weeks post-HBOT) and maintained at the ninth month, as confirmed by endoscopic assessment (SES-CD activity scale, p < 0.001). These findings bolster the potential of HBOT as a promising intervention in IBD management, modulating inflammatory factors and possibly influencing disease recurrence by promoting mucosal healing.
Approximately 20% of patients with long-standing IBD, especially those enduring its challenges for around 20 years, face a distressing complication: perianal fistulas, which significantly compromise their quality of life. Managing this complication requires an interdisciplinary approach, considering the low remission rates of fistulas and the high incidence of recurrence that often necessitates repeated surgical interventions. In this context, HBOT emerges as a potentially promising therapeutic option for addressing both inflammatory changes in the intestine and complications such as enterocutaneous or perianal fistulas. The therapeutic effects of HBOT are probably linked to the modulation of the immune response, making it a viable option, particularly for patients resistant to standard treatment regimens [13]. Our study also supports a combined therapy approach, yielding encouraging therapeutic outcomes. Notably, we observed a therapeutic effect persisting for about 9 months in terms of fistula healing, with all examined patients demonstrating regression of perianal lesions. These findings highlight the potential of HBOT as a valuable adjunctive intervention, especially in cases where conventional treatments are insufficient. This underscores its role in addressing challenging complications associated with IBD, ultimately enhancing patient outcomes and quality of life.
CD, when accompanied by perianal fistulas, profoundly impacts patients’ quality of life and is an ominous predictor of poor long-term outcomes [21]. The pathogenesis of CD-related perianal fistulas involves various factors, including the elevated production of transforming growth factor β, TNF, and IL-13 within the inflammatory infiltrate. This cascade triggers epithelial-to-mesenchymal transition and upregulates matrix metalloproteinases, leading to tissue remodeling and fistula formation. Addressing this complex condition effectively necessitates a multidisciplinary approach [22], a crucial aspect emphasised in our study. Our objective was to demonstrate the therapeutic impact of such an approach, focusing on promoting the healing of intestinal lesions and enhancing the efficacy of perianal fistula healing. Concurrent treatment with biological drugs has shown a tendency to strengthen the therapeutic effect [23, 24]. Although we could not statistically demonstrate this effect due to the small sample size, it is noteworthy for its potential in augmenting the benefits of the multidisciplinary treatment approach for CD complicated by perianal fistulas. Our study contributes to the growing body of evidence supporting the importance of a comprehensive and integrated treatment strategy in enhancing patient outcomes in this challenging subset of CD.
A systematic review and meta-analysis encompassing 16 studies, which included 164 patients with CD and various fistula forms (perianal, cutaneous-intestinal, and vaginal-intestinal), demonstrated a clinical response rate of 87% to HBOT, with clinical remission observed in 59% of cases. This analysis also highlighted a low risk of side effects associated with HBOT [25]. Recent research has been directed towards evaluating the effectiveness of polytherapy, which integrates conservative, surgical, and dietary approaches, each exhibiting variable effects [26]. Continued research is anticipated to significantly contribute to resolving this complex issue.
Conclusions
The application of combined therapy, enhanced with HBOT, resulted in remarkable clinical improvements in cases of CD complicated by perianal fistulas. Assessments using the CDAI revealed substantial clinical improvement, with an impressive 81.8% improvement rate. Clinical remission of the disease was observed in 54.5% of cases, as indicated by the SES-CD and the PDAI endoscopic activity scales.
Our study demonstrated statistically significant effects of HBOT on the SES-CD, PDAI, and calprotectin levels in stool, evident not only 6 weeks post-treatment but also in a follow-up assessment approximately 9 months later. Additionally, our findings suggest an enhanced therapeutic outcome through the concurrent use of biological treatment.
Importantly, our results indicate that HBOT holds substantial promise as a therapeutic option, particularly in medically challenging cases of CD characterised by recurrence and limited effectiveness of prior treatments. This modality offers hope for significant clinical improvement, with the potential to achieve remission rates exceeding 50%. The favourable outcomes observed in our study highlight the potential of HBOT in enhancing patient outcomes and may pave the way for further exploration and refinement of this treatment approach.
While HBOT is a safe and potentially effective treatment option for CD fistulas, randomised controlled trials are necessary to substantiate the benefits of this therapy in such cases.