Introduction
Coronary artery ectasia (CAE) is a variant of coronary artery disease characterized by abnormal dilation of the coronary arteries. CAE affects approximately 1% to 5% of all patients undergoing coronary angiography, indicating its notable but relatively uncommon presence in the broader context of coronary artery diseases [1, 2]. CAE is implicated in over 50% of cases of atherosclerosis, leading to clinical manifestations such as angina, vasospasm, and myocardial infarction [3, 4]. This condition complicates the management of acute coronary syndrome (ACS) due to its significant thrombus burden. Patients with CAE undergoing percutaneous coronary intervention (PCI) for ACS frequently face more complex procedures and an increased rate of adverse events [5].
The efficacy of excimer laser coronary angioplasty (ELCA) in managing ACS has been previously demonstrated, offering a promising approach to the challenges posed by complex PCI. ELCA has been particularly noted for its potential to facilitate favorable outcomes in cases with thrombus-laden lesions [6, 7]. However, the specific efficacy and safety of ELCA within this patient subset remain underexplored.
Therefore, this research aims to investigate the effectiveness of ELCA in enhancing reperfusion outcomes for patients with CAE-induced ACS.
Methods
Study population
We retrospectively investigated ACS patients who underwent primary PCI at Ogaki Municipal Hospital between January 2010 and December 2023, focusing on patients whose reports included the term ‘coronary ectasia’ or ‘ectatic coronary disease’. Coronary angiograms were obtained for angiographic analysis and were reviewed by two experienced observers to confirm the diagnosis of CAE. In each case, we measured the maximum diameter of the dilated artery and that of the healthy segments. CAE is defined as dilation of the coronary artery longer than 20 mm and ≥ 1.5 times the diameter of adjacent standard segments of the same or other arteries [8]. The measurements were made using QAngio XA (Version 7.3, Medis Medical Imaging System BV, Leiden, the Netherlands). Clinical data were retrieved from the hospital medical records. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and its amendments. This was reflected in the a priori approval of the study by the Ethics Committee of Ogaki Municipal Hospital. Written informed consent was obtained from all patients or their relatives before or after the PCI procedure.
Catheter procedures
ELCA was introduced at our institute in February 2016. The decision to use ELCA was made by the attending physician based on angiographic or intravascular ultrasound data. Excimer laser catheters are available in 0.9, 1.4, 1.7, and 2.0-mm diameters, with the 1.7-mm and 2.0-mm catheters offered in both concentric and eccentric configurations. In the present study, no patients underwent PCI with the 2.0-mm size. We utilized the Spectranetics CVX-300 platform (Spectranetics, Colorado, CO, USA), which includes an excimer laser generator (CVX-300) and a pulsed-wave xenon chloride excimer laser (X-80 Vitesse RX, Phillips Japan, Tokyo, Japan) emitting at a wavelength of 308 nm, with a pulse duration of 135 ns and a pulse output of 165 mJ/pulse. The patients underwent balloon dilation via standard techniques, and drug-eluting stents were deployed if necessary. The use of other devices such as thrombus aspiration devices was at the discretion of each physician. Thrombus characteristics were assessed through thrombus aspiration and categorized as red thrombus, white thrombus, or no thrombus retrieved. Antegrade flow before and after PCI was assessed using the thrombolysis in MI (TIMI) flow grading scale [9].
Statistical analysis
Continuous variables are expressed as median (minimum-maximum), and the characteristics of each group were compared using the Mann-Whitney U test. Categorical variables are expressed as numbers (percentages) and were compared using the χ2 test or Fisher’s exact test. A p-value of < 0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS, version 21.0 (IBM Corp.).
Results
During the study period, of the 3,222 ACS patients assessed, 21 (0.7%) presented with CAE and an initial TIMI flow of 0 or 1 and were ultimately included in the study. Of these, 6 (28.6%) patients underwent PCI using ELCA (ELCA group). The size of the laser catheter was 0.9 mm (n = 1), 1.4 mm (n = 1), 1.7 mm concentric (n = 2), and 1.7 mm eccentric (n = 2). The representative case is shown in Figure 1. The background characteristics of all patients are presented in Table I. The culprit lesion in ACS was identified in the right coronary artery (15 patients), left anterior descending artery (3 patients), and left circumflex artery (3 patients). Assessment of thrombus characteristics was performed in 16 patients who underwent thrombus aspiration. Thrombus characteristics were categorized as red thrombus (n = 7), and no thrombus retrieved (n = 4) in the non-ELCA group. Conversely, all cases in the ELCA group (n = 5) were categorized as red thrombus; in no patients was white thrombus retrieved. All patients in the ELCA group achieved a final TIMI flow of 2 or 3, compared to 53.4% in the non-ELCA group (p = 0.04). During the follow-up period, all patients in the ELCA group underwent follow-up coronary angiography (CAG) or coronary computed tomography angiography (CCTA) between 3 and 876 days post-procedurally. No significant restenosis was observed in this group. Conversely, in the non-ELCA group, 11 patients underwent follow-up CAG or CCTA between 3 and 384 days post-procedurally, with 3 (27.3%) patients exhibiting significant restenosis.
Figure 1
Representative case. A – Angiography showing total occlusion of the proximal portion of the right coronary artery. B – After thrombus aspiration. C – Intravascular ultrasound (IVUS) revealing a large amount of thrombus and dilated coronary artery ectasia. D – Excimer laser coronary angioplasty (ELCA) on the culprit lesion. E – After ablation using ELCA, the amount of thrombus was reduced. F – IVUS findings after ELCA showing reduced thrombus (dashed line). G – Dilation with a balloon. H – Final coronary angiography after intra-aortic balloon pumping assistance. I – Three days after intervention
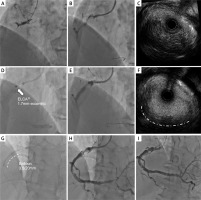
Table I
Patient characteristics
| Parameter | All N = 21 | Non-ELCA group n = 15 (71.4%) | ELCA group n = 6 (28.6%) | P-value |
|---|---|---|---|---|
| Age [years] | 61 (38–89) | 68 (45–89) | 56 (38–62) | 0.07 |
| Male, n (%) | 19 (90.5) | 13 (86.7) | 6 (100) | 0.35 |
| Body mass index [kg/m2] | 26.0 (20.9–33.8) | 25.6 (20.9–31.9) | 28.7 (24.6–33.8) | 0.07 |
| Past history, n (%): | ||||
| Hypertension | 13 (61.9) | 8 (53.3) | 5 (83.3) | 0.20 |
| Atrial fibrillation | 3 (14.3) | 2 (13.3) | 1 (16.7) | 0.84 |
| Diabetes mellitus | 5 (23.8) | 3 (20.0) | 2 (33.3) | 0.52 |
| Previous PCI | 1 (4.8) | 0 (0) | 1 (16.7) | 0.11 |
| Dyslipidemia | 16 (76.2) | 12 (80.0) | 4 (66.7) | 0.52 |
| Current smoker | 10 (47.6) | 7 (46.7) | 3 (50.0) | 0.89 |
| PCI procedural findings: | ||||
| Diagnosis, n (%): | 0.11 | |||
| STEMI | 14 (66.7) | 12 (80.0) | 2 (33.3) | |
| Non-STEMI | 4 (19.0) | 2 (13.3) | 2 (33.3) | |
| UAP | 3 (14.3) | 1 (6.7) | 2 (33.3) | |
| Culprit lesion, n (%): | 0.33 | |||
| RCA | 15 (71.4) | 10 (66.7) | 5 (83.3) | |
| LAD | 4 (19.0) | 4 (26.7) | 0 (0) | |
| LCX | 2 (9.5) | 1 (6.7) | 1 (16.7) | |
| ACC/AHA classification of coronary lesions, n (%): | 0.48 | |||
| B1 | 2 (9.5) | 1 (6.7) | 1 (16.7) | |
| B2 | 19 (90.5) | 14 (93.3) | 5 (83.3) | |
| Length of CAE [mm] | 31.3 (25.0–44.0) | 31.3 (27.7–38.2) | 32.4 (22.4–48.4) | 0.94 |
| Ratio of CAE* | 1.8 (1.6–2.2) | 1.8 (1.6–2.3) | 1.8 (1.6–1.9) | 0.67 |
| Initial TIMI flow, n (%): | 0.84 | |||
| 0 | 18 (85.7) | 13 (86.7) | 5 (83.3) | |
| 1 | 3 (14.3) | 2 (13.3) | 1 (16.7) | |
| Final TIMI flow, n (%): | 0.15 | |||
| 0 | 3 (14.3) | 3 (20.0) | 0 (0) | |
| 1 | 4 (19.0) | 4 (26.7) | 0 (0) | |
| 2 | 3 (14.3) | 1 (6.7) | 2 (33.3) | |
| 3 | 11 (52.4) | 7 (46.7) | 4 (66.7) | |
| Final TIMI flow 2 or 3, n (%) | 14 (66.7) | 8 (53.4) | 6 (100) | 0.04 |
| Size of laser catheter, 0.9/1.4/1.7/1.7-E, n | – | – | 1/1/2/2 | |
| IVUS, n (%) | 14 (66.7) | 8 (53.3) | 6 (100.0) | 0.04 |
| Aspiration, n (%) | 16 (76.2) | 11 (73.3) | 5 (83.3) | 0.63 |
| Thrombus characteristics, n (%) (n = 16)**: | 0.12 | |||
| Red thrombus | 12 (75.0) | 7 (63.6) | 5 (100) | |
| White thrombus | 0 (0) | 0 (0) | 0 (0) | |
| No thrombus retrieved | 4 (25.0) | 4 (36.4) | 0 (0) | |
| Distal protection, n (%) | 1 (4.8) | 1 (6.7) | 0 (0) | 0.52 |
| Non-stenting, n (%) | 8 (38.1) | 7 (46.7) | 1 (16.7) | 0.20 |
| Balloon size [mm] | 4.0 (1.25–5.5) | 4.0 (1.25–5.0) | 3.5 (2.0–5.5) | 0.44 |
| IABP, n (%) | 9 (42.9) | 6 (40.0) | 3 (50.0) | 0.68 |
| Medical treatment, n (%): | ||||
| OAC | 8 (38.1) | 4 (26.7) | 4 (66.7) | 0.09 |
| Aspirin | 16 (76.2) | 13 (86.7) | 3 (50.0) | 0.08 |
| P2Y12 inhibitors | 15 (71.4) | 10 (66.7) | 5 (83.3) | 0.45 |
| Urokinase | 0 (0) | 0 (0) | 0 (0) | > 0.99 |
* The ratio is calculated as maximum CAE diameter / diameter of adjacent standard segments of the same or other arteries.
CAE – coronary artery ectasia, ELCA – excimer laser coronary angioplasty, IABP – intra-aortic balloon pumping, IVUS – intravascular ultrasound, LAD – left anterior descending artery, LCX – left circumflex artery, OAC – oral anticoagulant, PCI – percutaneous coronary intervention, RCA – right coronary artery, STEMI – ST-elevated myocardial infarction, TIMI – Thrombolysis In Myocardial Infarction, UAP – unstable angina pectoris.
Discussion
Our results demonstrated that PCI using ELCA led to improved TIMI flow in all instances. Notably, patients treated with ELCA did not experience subsequent restenosis, suggesting the potential utility of this technique in this specific patient cohort. While it may be challenging to compare these results with patients from the era before ELCA was available, these findings underscore the significance of ELCA in managing severe ACS presentations associated with CAE. However, the small sample size limits the generalizability of our results, and future research with larger cohorts is warranted.
The pathogenesis of CAE poses significant challenges in clinical management, particularly with ACS. CAE is primarily associated with exaggerated expansive remodeling, driven by enzymatic degradation of the coronary artery’s medial layer. This process is typical of atherosclerosis, involving lipoprotein accumulation, oxidative stress, inflammatory cell infiltration, altered nitric oxide metabolism, and reduced shear stress. These factors lead to the breakdown of medial collagen and elastin, disrupting the elastic laminae of coronary arteries [10, 11]. Even without significant stenosis, patients with CAE can experience chest symptoms due to reduced coronary flow reserve and microcirculatory dysfunction [12]. The implementation of PCI in CAE patients further complicates clinical outcomes. The challenges of PCI in this context include selecting appropriate stent sizes due to enlarged vessel diameters, excessive thrombus complicating stent placement, and turbulent flow in ectatic arteries that increase restenosis risk. These factors necessitate a careful and tailored approach to the management of CAE, highlighting the need for innovative treatment strategies to address the unique complexities of this condition.
ELCA operates on the principles of photomechanical, photochemical, and photothermal energies released by catheter-guided pulsed ultraviolet excimer laser light. This technology effectively evaporates thrombus and plaque, thereby contributing to targeted volume reduction [13, 14]. In CAE, stagnant blood flow and reduced shear stress facilitate thrombus formation, triggering acute coronary syndrome. Using ELCA can vaporize thrombus and reduce its volume, potentially improving blood flow. The vaporization of the thrombus and plaque can enhance the effectiveness of subsequent interventions, such as thrombus aspiration and balloon angioplasty. Furthermore, the ‘stunned platelets’ phenomenon, characterized by decreased platelet aggregation and reduced platelet force development capability, contributes to the antithrombogenic effects observed with ELCA [15]. These properties may explain the improved outcomes in our study and suggest a particularly promising role for ELCA in managing patients with CAE, where traditional strategies may fall short.








