Summary
Pulmonary hypertension has been a quite common phenomenon in patients undergoing transcatheter aortic valve replacement (TAVR), and appears to be independently associated with acute kidney injury (AKI) following the TAVR procedure. In this context, a better comprehension of pathophysiological mechanisms associated with AKI evolution might help establish effective reno-protective strategies in both the pre- and post-TAVR settings.
Introduction
Over recent years, transcatheter aortic valve replacement (TAVR) has been a widely recognized management strategy for surgically high-risk or inoperable subjects with symptomatic severe valvular aortic stenosis (AS) [1, 2]. Acute kidney injury (AKI) has been a frequently encountered phenomenon following TAVR (incidence ranging from 3% to 50%) possibly relying on the existing comorbidities, the amount of contrast medium, the necessity for rapid pacing leading to hypotension, and postprocedural challenges including access site complications [3, 4]. AKI following TAVR might be strongly associated with unfavorable short- and long-term prognosis, increased length of hospital stay, etc. [5]. In this context, it seems crucial to identify patients at high risk for AKI following TAVR. Therefore, there is a strong necessity to harness easily applicable, simple, and inexpensive predictors of AKI in this setting.
Transthoracic echocardiography (TTE) has been a common screening modality primarily used to evaluate patients with cardiovascular symptoms. Pulmonary hypertension (PH) detected on TTE (as determined with an increased pulmonary artery systolic pressure (PASP) value) [6] might be caused by a variety of conditions, and might have a strong link with an unfavorable prognosis irrespective of the underlying condition [7]. Over recent years, most studies have focused on the potential association of AKI with percutaneous coronary intervention (PCI). However, there has been little emphasis on TAVR-related AKI. Interestingly, the amount of contrast media used is lower in TAVR compared with PCI. Of note, increased PASP might serve as a potential trigger of AKI largely through induction of right heart failure and systemic congestion with consequent reduction in transglomerular pressure and kidney perfusion [8].
Aim
Accordingly, the present study aims to assess the effects of PASP on the evolution of AKI in patients undergoing TAVR in an effort to ameliorate risk stratification in this expanding population.
Material and methods
Study population
One hundred and two consecutive subjects with symptomatic severe valvular AS undergoing TAVR between January 2010 and May 2022 were included in this retrospectively designed study. Procedure details, characteristics and follow-up data were gathered and evaluated. Exclusion criteria were as follows: significant involvement in other cardiac valves (except tricuspid regurgitation); severe left ventricular dysfunction (defined as a left ventricular ejection fraction of ≤ 30%), presence of other systemic conditions and functional class IV. Subjects who did not meet the inclusion criteria and those who died within the first 24 h following the procedure were also excluded. Finally, 90 participants were included in this analysis. Subjects were categorized into two groups based on the emerging TAVR-related AKI. AKI was defined as a 25% increase in serum creatinine values within 72 h (or an absolute increase of 0.5 mg/dl) following TAVI compared with baseline creatinine values.
The study protocol was endorsed by the Ethical Committee of the local university and performed in line with the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of the analysis.
Echocardiography
A blinded experienced operator collected the whole data. Philips Affiniti 50 (Philips Medical Systems, Andover, MA) or GE Vivid-q (GE Vingmed Ultrasound AS, Horten, Norway) was used for basic echocardiographic analysis. Gradients and dimensions were analyzed with standard methods. A PASP value of > 35 mm Hg was accepted as the presence of PH. A PASP value of ≥ 50 mm Hg was accepted as the presence of moderate-severe PH [9].
Procedure
Deep sedation or general anesthesia was the preferred strategy during TAVR. Temporary pacemaker implantation mostly through a transfemoral access was performed for rapid pacing and for possible atrioventricular block. Initially, two Proglides were inserted for percutaneous closure following the intervention. The following self-expandable aortic bioprostheses were implanted: CoreValve (Medtronic, Minneapolis, Minnesota, United States), Portico (Abbott Structural Heart, St Paul, MN, USA) or ACURATE neo aortic valve (Boston Scientific, Marlborough, MA, USA). Following deployment of the device, a potential paravalvular leak was further analyzed with contrast injection. Repeat angiography of the femoral area was also implemented to evaluate vascular integrity, possible extravasation and vascular dissection, etc. Following the procedure, all patients underwent transthoracic echocardiography for the evaluation TAVR device functions and potential subclinical complications (pericardial effusion, etc.).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics software, version 21 (IBM Corp, Armonk, NY, USA). Mean ± standard deviation, or median [interquartile range], were used to express continuous data that were compared with Student’s t test or the Mann-Whitney U test, where appropriate. Percentage values and frequencies were used to express categorical variables that were compared with the Fisher exact test or Pearson χ2 test. Predictors of AKI were determined using multivariable logistic regression. Statistical significance was accepted as p < 0.05. Predictive power of PASP was analyzed using the receiver operating characteristic (ROC) curve.
Results
A total of 90 subjects (56.7% female, with a mean age of 79.2 ±7.5 years) with severe, symptomatic aortic stenosis (mean transaortic pressure gradient, P mean 46 ±16 mm Hg), and high surgical risk (mean logistic EuroSCORE of 14.6 ±9.4%) constituted this retrospective cohort study. In the study population, the overall incidence of AKI was 25.6%.
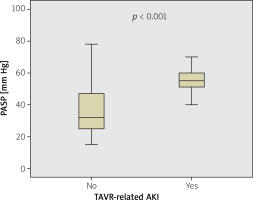
Characteristics of the two groups (at baseline) are presented in Table I. No significant diversities in baseline clinical data exist between the two groups, except baseline PASP, white blood cell counts and EuroSCORE. The mean contrast media volume during the procedure was 152 ±65 ml, and none of the patients underwent percutaneous coronary intervention during the index TAVR procedure. In terms of baseline PASP, the TAVR-related AKI (+) group had higher values of PASP compared with the AKI (–) group (55.4 ±14.0 vs. 37.1 ±16.3 mm Hg, p < 0.001) (Table I, Figure 1).
Table I
Comparison of data of patients with and without acute kidney injury in TAVI patients
Figure 1
Comparison of pulmonary artery systolic pressure (PASP) levels according to the development of transcutaneous aortic valve replacement (TAVR)-related acute kidney injury (AKI) or not
The independent AKI predictors were EuroSCORE (OR = 1.238, 95% CI: 1.093–1.401, p = 0.001), PASP (OR = 1.076, 95% CI: 1.017–1.139, p = 0.011), and systemic arterial hypertension (OR = 3.544, 95% CI: 1.438–5.738, p = 0.017) in the multivariate logistic regression analysis (Table II).
Table II
Multivariate logistic regression analysis of potential predictors for acute kidney injury
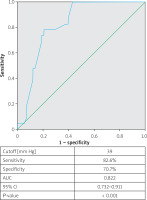
In ROC curve analysis, a PASP value of > 39 mm Hg was found to predict TAVR-associated AKI (yielding specificity and sensitivity values of 70.7% and 82.6%, respectively) (AUC = 0.822; p < 0.001) (Figure 2).
Discussion
This study has demonstrated the clinical value of admission PASP in subjects with severe valvular AS undergoing TAVR. Importantly, the pre-procedural value of PASP was found to be independently associated with the evolution of TAVR-related-AKI (Figure 3). In this context, a higher PASP value might serve as a potential marker of AKI in these subjects.
Over recent years, there have been significant advances in TAVR in terms of interventional experience, collaboration among clinical teams, and. more importantly. evolution of the devices [10]. Therefore, TAVR is currently accepted as the preferred strategy for subjects with severe AS having high or intermediate operational risk [11]. However, peri-procedural complications might also pose a significant challenge in certain settings. Among these, AKI has been regarded as one of the most frequent and life-threatening complications in subjects undergoing TAVR [12–15]. On the other hand, little is known regarding the predictors of renal dysfunction in subjects with severe AS undergoing TAVR. In the pre-TAVR setting, identification of patients who might be prone to the development of procedure-related AKI might help tailor patient-specific management strategies.
Several studies have previously suggested hemodynamic and contrast-associated factors as the fundamental triggers of AKI evolution [16, 17]. Ozbeyaz et al. [18] investigated the potential association between the H2FPEF score (comprising PH, obesity, hypertension, atrial fibrillation, age of > 60 years, and E/e′ > 9) and AKI in subjects undergoing PCI. The authors suggested H2FPEF score as an independent risk factor for AKI evolution. Notably, there has been no single study to date exclusively focusing on the impact of PASP on AKI evolution following TAVR. Importantly, multivariate analysis suggests PASP as an independent predictor of TAVR-related AKI. Therefore, our study might provide new insights in the setting of TAVR-related AKI.
On the other hand, the precise mechanisms of how high PASP values might lead to TAVR-related AKI still need to be elucidated. However, several potential mechanisms have been postulated. It is well known that PH together with enhanced pulmonary vascular resistance also gives rise to significant increases in the RV pressure and wall tension. In this context, hemodynamic destabilization of the RV, even in a subtle manner, might potentially cause a significant decrease in left ventricular preload with a consequent reduction in stroke volume with or without systemic hypotension [19]. Moreover, RV failure might also lead to a state of systemic congestion and enhanced central venous pressure and elicit variable degrees of stagnation and hyperemia in central veins of the liver and kidneys [8]. These mechanisms ultimately lead to a significant reduction in trans-glomerular pressure gradients and potentially impair renal perfusion. Apart from the gross impact of circulatory failure, activation of various neurohormonal cascades (including renin-angiotensin-aldosterone, sympathetic and natriuretic peptide systems) also significantly contribute to the renal injury through molecular mechanisms including oxidative stress. On the other hand, compensatory neurohormonal activation mostly in response to reduced cardiac output might further enhance systemic venous congestion and hence might further increase the extent of renal injury, potentially creating a vicious cycle in this context [20–22]. Importantly, these alterations might already exist in the pre-TAVI setting, mostly in a subclinical manner. However, the additive impact of certain factors associated with the TAVR procedure (including the impact of contrast media and anesthetic agents, and rapid pacing) might lead to substantial RV failure in the post-TAVI setting. Taken together, it might be suggested that since pre-existing PH might significantly impair the physiological reserve of the RV and systemic compensatory mechanisms, it might significantly reduce the threshold of AKI evolution associated with TAVR-related factors. In this context, a recent study has demonstrated higher PASP values in patients complicated by AKI in the post-TAVR setting, suggesting PASP as a potential determinant of AKI evolution following TAVR based on the above-mentioned mechanisms [23]. Interestingly, none of the well-known AKI scores worked perfectly in the prediction of AKI evolution following TAVR in this study [23].
In line with previous studies, the present study also suggests that EuroSCORE and systemic hypertension may be associated with AKI evolution [24, 25]. We hold the opinion that this association might be ascribed to the potential adverse effects of hypertension and other co-morbidities on physiological renal reserve (including arteriolar autoregulation).
Study limitations. The present study has a variety of inherent limitations. First, this study was a retrospective and single-center analysis. Second, the analysis had a relatively small sample size that might preclude drawing firm conclusions on the investigated issue. Third, evaluation of serum creatinine values might have been affected by potential confounders (severe hemodynamic compromise, nephrotoxic drugs, etc.), which could independently contribute to AKI, regardless of pre-existing PH.
Conclusions
PH has been a quite common phenomenon in patients undergoing TAVR, and appears to be independently associated with AKI following the TAVR procedure. In this context, a better comprehension of pathophysiological mechanisms associated with AKI evolution might help establish effective reno-protective strategies in both the pre- and post-TAVR settings.