Introduction
An important issue in the context of the pathophysiology of irritable bowel syndrome (IBS) is lactose intolerance. It has been repeatedly proven in the world literature, that IBS patients more often report symptoms of lactose intolerance compared to healthy people [1]. However, only in about 2/3 of patients reporting symptoms of lactose intolerance, such as abdominal distension, excessive gas production, feeling overflowing, vomiting, or diarrhoea, is lactase deficiency confirmed by the hydrogen breath test [2].
Complaints related to lactose intolerance may overlap with the symptoms of IBS or lead to the misdiagnosis of IBS. It is therefore extremely important to make a correct diagnosis [3] because, unlike functional disorders such as IBS, lactase deficiency affects digestive processes and nutrient absorption. In addition, targeted treatment (elimination diet or lactase enzyme supplementation) in patients with hypolactasia leads to rapid clinical improvement.
Differences in the activity of the lactase enzyme between individuals are due to genetic polymorphism. Primary adult lactase deficiency (adult type hypolactasia) is the most common type of primary enzyme deficiency and determines the decline in the activity of the intestinal lactase enzyme with age, beginning from 4–5 years of age. The prevalence of adult type hypolactasia is estimated at 65–70% [4]. Primary hypolactasia is based on changes in a single autosomal MCM6 (minichromosome maintenance complex component 6) gene, located on the second chromosome (2q21), in the promoter region of the lactase gene (LCT). In Europe, 2 polymorphisms within this gene are responsible for the reduction of lactase production: C/T-13910 and G/A-22018 [5]. The TT and AA variants are dominant and determine the maintenance of lactase activity at a high level, also in adults, while the CC and GG variants are responsible for the occurrence of adult type hypolactasia; in CT and AG heterozygotes, lactase activity shows intermediate values and is variable [6–8]. In adults with the TT genotype, a tenfold higher level of lactase was detected in the brush border biopsy of the small intestine compared to patients with the CC genotype – a homozygous form of hypolactasia [9]. In heterozygous individuals with intermediate lactase activity increases the susceptibility to the development of lactose intolerance.
The incidence of lactose intolerance in IBS varies widely in the literature (27–72%) [10, 11].
Material and methods
The study included 56 patients with IBS (45 women and 11 men; mean age: 41.2 ±14.4 years) diagnosed based on the Rome III criteria (patients with predominant diarrhoea (IBS-D) – 51.8% (n = 29), with predominant constipation (IBS-C) – 28.6% (n = 16), with mixed/unclassified type of IBS – 19.6% (n = 11)). The control group consisted of 23 healthy people (13 women, 10 men; mean age 38.56 ±16.61 years). The exclusion criteria were the use of antibiotics or laxatives (including preparation for colonoscopy) within the 4 weeks prior to HBT and the coexistence of organic gastrointestinal diseases.
The study was approved by the Bioethics Committee of the Medical University of Lodz on 20 November 2012 – approval No. RNN/214/12/KE.
All study participants, both IBS patients and healthy controls, were asked to complete a questionnaire on IBS symptoms and tolerance of lactose-containing products.
All subjects underwent a hydrogen breath test (HBT) with 50 g of lactose (dissolved in 200 ml of water) using a GastrolyzerGastro+® (Bedfont) device. The first measurement of exhaled hydrogen was made before consumption of the lactose solution, and then after it was consumed, at 30-minute intervals, for a total of 240 min. An increase in the hydrogen level in the exhaled air by > 20 ppm compared to the initial value was considered a positive result of the HBT. An increase in hydrogen in the exhaled air after at least 60 min from the start of the test by 20–40 ppm indicated a mild deficiency of the lactase enzyme, an increase between 40 and 80 ppm – a moderate enzyme deficiency, and over 80 ppm – a severe deficiency of the lactase enzyme [12]. In the group of patients with positive results of the hydrogen test, indicating deficiency of the lactase enzyme, the polymorphism C/T -13910 and G/A -22018 in the promoter of the LCT gene encoding lactase was determined. The determination of polymorphisms in the LCT gene was performed in blood serum using a multiplex PCR reaction with reverse hybridization; the tests were carried out in a commercial laboratory using the GenoType LCT test (Hain Lifescience GmbH, Nehren, Germany).
Statistical analysis
Statistical analysis were performed using Statistica 10 (StatSoft, Tulsa, USA). Two independent groups were compared in the analysed study. Nominal variables are presented as percentages. To compare the 2 nominal variables, the χ2 test with Yates’s correction or the exact 2-tailed Fisher test was used, depending on the size of the study groups. The level of statistical significance was p < 0.05.
Results
In the study, based on the results of the hydrogen breath test with lactose solution, lactase deficiency was diagnosed in 34 (60.7%) patients with IBS, and in 10 (43.5%) patients in the control group. The differences in the reported values between the study groups were not statistically significant. The results of the hydrogen breath test in individual types of IBS were also analysed. There were no statistically significant differences between the incidence of lactase deficiency in particular subgroups of patients (IBS-D 62.1%, IBS-C 56.3%, IBS mixed/unclassified 63.6%).
In the group of IBS patients with abnormal HBT results, mild lactase deficiency was found in 4 (11.8%) patients, moderate in 8 (23.5%), and severe in 22 (64.7%). Therefore, significantly more often lactase deficiency was severe than moderate and mild (p < 0.05). In the control group there were no cases of mild lactase deficiency, severe lactase deficiency occurred in 7/10 patients, and in the remaining 3 the lactase deficiency was moderate. The differences between the frequency of moderate and severe lactase deficiency were not significant in the control group. There were no statistically significant differences in the frequency of lactase deficiency of particular severity between the study group and the control group.
C/T-13910 and G/A-22018 polymorphisms in the promoter of the LCT gene encoding lactase were determined in 38 patients with positive results of the hydrogen test. Primary adult type hypolactasia, genetically determined (variant C/C at position -13910 and /or variant G/G at position -22018 of the LCT gene), was confirmed in 78.9% (n = 30; 23 – study group, 7 – control group). In the remaining 8 (6 – study group, 2 – control group) the heterozygous C/T-13910 and G/A-22018 system was found in the promoter of the LCT gene, which determines the indirect activity of lactase (Table I).
Table I
The results of the genetic study of the C/T-13910 and G/A-22018 polymorphisms in the promoter of the LCT gene among patients with a positive HBT result
No T/T-13910 or A/A-22018 homozygous variants were detected in any of the HBT-positive patients (both study and control group), the presence of which would exclude the genetic basis of lactase deficiency. In the study group and in the control group, the percentage of patients with genetically determined lactase deficiency was similar (79.3% vs. 77.8%).
Eight patients with lactase deficiency found in the HBT (6 in the study group and 2 healthy people) were heterozygous in the genetic test, which determines the intermediate activity of lactase. In this group, 3 patients were diagnosed with SIBO (patients with IBS), so hypolactasia was probably of a secondary nature. In the remaining 5 patients, mild or moderate lactase deficiency detected in the HBT did not cause severe symptoms of lactose intolerance and was not clinically significant.
There were also no statistically significant differences in the occurrence of LCT gene polymorphisms in particular IBS subtypes (IBS-D 73.3%, IBS-C 85.7%, IBS mixed/ unclassified 85.7%). Analysing the results of genetic studies of polymorphism in the LCT gene, it was found that adult hypolactasia is significantly more common in patients with severe than moderate and mild enzyme deficiency in HBT (p < 0.05) (Figure 1).
Figure 1
Incidence of adult type hypolactasia genetically confirmed according to the severity of lactase deficiency at the HBT
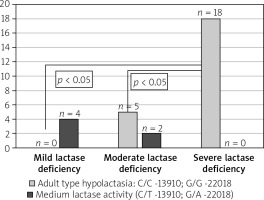
The analysis of complaints reported by patients during and after the hydrogen breath test showed no statistically significant differences between the study and control groups. Adult type hypolactasia was confirmed in 78.9% (n = 15) of IBS patients who reported symptoms of lactose intolerance during the HBT and had the C/T-13910 and G/A-22018 polymorphism. In the control group, all patients reporting symptoms of lactose intolerance during the HBT had genetically confirmed hypolactasia. However, the differences between the IBS group and the control group were not statistically significant (p > 0.05).
Discussion
In our study, genetically determined adult type hypolactasia (variant CC at position -13910 and/or variant GG at position -22018 of the LCT gene) was confirmed in 78.9% (n = 30) of patients (both IBS patients and healthy subjects) with a positive hydrogen test, indicating lactase enzyme deficiency. The remaining 8 HBT-positive patients had a heterozygous C/T-13910 and G/A-22018 system in the promoter of the LCT gene, which determines indirect lactase activity; T/T-13910 or A/A-22018 homozygous variants were not detected. The frequency of the C/T-13910 and G/A-22018 genotypes in the LCT gene promoter did not differ significantly in the IBS patients and the control group. In the studies published so far assessing the compliance of clinical symptoms of lactose intolerance with the results of genetic studies of the C/T-13910 and G/A-22018 polymorphisms in the promoter of the LCT gene, divergent results were obtained. According to Bodlaj et al. [13], only 50% of patients with positive HBT results showed the CC genotype. On the other hand, in the studies by Hoegenauer et al. [14] and Ridefelt et al. [15], the presence of the CC genotype was genetically confirmed in more than 90% of patients with lactose intolerance. In the study by Bernardes-Silva et al. variant C/C-13910 was identified in 100% of patients with lactose intolerance, and G/G-22018 in 96%. It was also found that the presence of the C or G allele was associated with a significantly higher level of hydrogen in the exhaled air during the HBT and with a greater intensity of symptoms of lactose intolerance [16].
Among the patients who reported symptoms of lactose intolerance during the HBT, in the group of IBS patients, genetically determined adult hypolactasia was confirmed in 78.9% of patients (n = 15), and in the control group – in 100%. This result may support the theory that in patients with IBS, lactose tolerance is affected not only by the activity of intestinal lactase, but also by other factors such as visceral hypersensitivity. The relationship between the C/T-13910 and G/A-22018 polymorphisms in the promoter of the LCT gene with IBS symptoms in particular disease subtypes was investigated by Kumar et al. [17]. Patients with IBS-D constituted 52% subjects in that study, IBS-C – 35%, and the mixed and unclassified form – 13% (according to Rome III criteria). Similarly, in the group analysed by us, patients with diarrhoea constituted 51.8%, patients with constipation – 28.6%, and mixed/unclassified patients – 19.6%. In the study by Kumar et al. the frequency of individual genotypes did not differ between the study group and the control group. However, they showed significantly more frequent occurrence of CC and GG genotypes among patients with IBS-D compared to other IBS subtypes and the control group. Patients with IBS and the CC or GG genotype significantly more often reported abdominal pain, flatulence, and diarrhoea after consuming lactose-containing products, compared to patients with AA and TT genotypes or heterozygous [17].
In the study by Almazar et al., in patients with IBS (diagnosed according to the Rome II Criteria), it was again shown that the frequency of the CC genotype does not differ between IBS patients and the control group (14.8% vs. 15.1%) [18]. However, the frequency of subjectively reported lactose intolerance among IBS patients was significantly higher than in healthy subjects (45.0% vs. 9.8%). Lactose intolerance was reported more often in patients with the diarrhoeal form (57.7%) compared with the other subtypes (constipation – 36.2%, mixed – 46.3%), although there were no statistically significant differences in the frequency of CC genotypes between individual subtypes. In all HBT-positive patients, lactase deficiency was confirmed in this study with genetic testing.
In our analysis, 8 patients with lactase deficiency detected with HBT (6 IBS and 2 control) were heterozygous. In this group, 3 patients showed the features of small intestinal bacterial overgrowth, so the lactase deficiency could be secondary in them, while in the remaining 5 patients, mild or moderate lactase deficiency detected in the HBT did not cause symptoms of lactose intolerance, so it had no clinical significance. It is also worth emphasizing that a homozygous TT or AA allele system was not found in any of the subjects with a positive HBT result in the study group, excluding primary lactase deficiency in this group of patients.
On the other hand, in an Italian study [19] conducted in a group of 32 healthy subjects, a positive HBT result was obtained in 88.5% of patients with the CC genotype, and there was no positive result in the heterozygous (CT) group. However, among patients with the CT genotype, despite negative HBT results, there were cases of symptoms of lactose intolerance. In this study the results of the genetic test and the HBT were more compatible in patients over 30 years of age. The authors suggest that these tests can be treated as complementary, and the selection of each of them should be made depending on the clinical symptoms of individual patients.
We found that adult type hypolactasia is significantly more common in patients with severe than moderate and mild enzyme deficiency, which explains the greater severity of lactose intolerance in patients with genetically confirmed hypolactasia. This is confirmed by the results of a study conducted in Sweden, which showed that patients with the CC genotype during the HBT showed significantly more symptoms of lactose intolerance than people with the CT and TT genotypes [20]. Moreover, although statistically insignificant, abdominal pain during the HBT was observed more often in CT heterozygotes than TT homozygotes. The HBT results were significantly more likely to be positive in the group of patients with the CT genotype than TT homozygotes, but no significant differences were found in the frequency of IBS among the groups of patients with particular genotypes.
We consider it a potential bias of our study that genetic tests were not performed in all patients, only in those with positive HBT. Therefore, our analysis does not allow us to determine the total frequency of hypolactasia in the studied population.
Conclusions
The incidence of lactase deficiency in IBS patients is not different from that found in healthy subjects. Nevertheless, irrespective of the IBS subtype, lactose intolerance poses a significant clinical problem. Lactase deficiency is detected in more than half of IBS patients. In addition, in more than three-quarters of patients with lactase deficiency (both IBS patients and healthy people), hypolactasia is confirmed genetically. IBS patients are more likely to report symptoms of lactose intolerance than healthy subjects. It seems, therefore, that the subjective feelings of patients are due to the visceral hypersensitivity rather than the real lactase deficiency. However, in patients diagnosed with severe lactase deficiency based on the HBT, there is a high probability of genetically determined hypolactasia. This group of patients often with non-characteristic clinical symptoms, needs to be distinguished from IBS due to the possible targeted management, i.e. benefit from introducing a lactose-free diet or lactase supplementation.










