Introduction
Cortisol, the main glucocorticoid hormone in humans, is the effector of the hypothalamus-pituitary-adrenal (HPA) axis. It is secreted in the circadian rhythm, with its peak in the morning and nadir in the evening [1]. This pattern of cortisol release is established in infancy, as early as around one-month-old, and is present throughout life [2]. Salivary cortisol (SC) is a feasible marker for the evaluation of HPA axis activity, especially its response to stress, due to the non-invasive method of sample collection. Salivary cortisol reflects the level of biologically active free serum cortisol, so it is unaffected by the level of cortisol binding globulin (CBG) or albumin [3]. Due to lipid solubility the SC concentration is also independent of saliva flow.
It is postulated that excessive activation of the HPA axis in early life can induce a long-term influence on its function. Neonates in intensive care units (ICU) experience recurrent episodes of stress due to painful medical procedures [4]. As a result, they are potentially prone to dysregulation of the HPA axis. The analysis by Ivars et al., including 51 preterm infants, revealed slightly lower median values of morning SC concentrations at the expected date of delivery (DED) in comparison to healthy term infants [5]. The persistence of differences in morning SC between preterm and term infants was observed at 3, 8, and 18 months of corrected age (CA) [6, 7]. Although those findings indirectly suggest the influence of repeated stress on the HPA axis, to date only one study showed a direct correlation between the number of skin-breaking procedures experienced by preterm newborns and morning SC [8]. So far, the impact of repetitive stress on morning SC in term neonates hospitalized in the ICU has not been analysed.
Aim of the study
The aim of the present study was to evaluate the influence of repeated pain exposure on morning SC in newborns admitted to the ICU. The other objective was to analyse the potential impact of perinatal factors, especially exposure to antenatal corticosteroid prophylaxis and degree of prematurity, on HPA axis activity.
Material and methods
Study population
Newborns were recruited from a tertiary referral Neonatal Intensive Care Unit between June 2018 and March 2021. The neonates were divided into 3 groups based on their gestational age (GA): term (370/7–416/7 weeks), moderate to late preterm (320/7–366/7 weeks), and very preterm (< 320/7 weeks). The exclusion criteria included occurrence of major malformation, clinically suspected genetic disorder, severe intraventricular haemorrhage (IVH grade IV), congenital adrenal hyperplasia, history of any surgical procedure, and earlier hospitalization in neonatology units longer than 3 days.
Study design
Patients’ medical records were analysed for perinatal history. In each patient, the hospital stay was prospectively monitored for the duration of mechanical ventilation, non-invasive ventilatory support, and the number of the most common medical procedures including intubation, invasive blood sampling (though venipuncture or heel lance), urinary bladder catheterization, eye examination, lumbar punction, ultrasound (each body part considered as separate examination), vaccination, peripheral and central vein canulation, arterial line insertion, and umbilical artery/vein cannulation. The administration of local and systemic corticosteroids was analysed. Saliva samples for morning SC were collected after completion of 35 weeks of postmenstrual age (PMA) in preterm infants and before discharge in term neonates. Premature infants were qualified for saliva sampling if they were in a stable medical condition defined as the absence of invasive ventilation or cardiovascular support. Otherwise, the analysis was postponed until the criteria were fulfilled. Saliva collection was repeated on different days. At least 2 samples were obtained from each patient.
Saliva sampling
All samples were collected in the morning between 6 and 9 a.m., before any other nursing or diagnostic procedures, with at least a one-hour interval from feeding, without using saliva stimulants. Before collection, a gentle examination of the oral cavity was performed to decrease the risk of contamination with milk. Samples were collected from the floor of the mouth and buccal mucosa using SalivaBio Infant’s Swabs (SIS) (Salimetrics LLC, State College, PA, USA). If milk reflux appeared, sampling was stopped and postponed. The maximum collection time was 10 minutes. After sampling saliva was pressed out from SIS into Eppendorf Tubes (1.5 ml) using a syringe. All samples were kept frozen at –20°C until their processing.
Salivary cortisol measurements
Salivary cortisol concentrations were measured using radioimmunoassay (Cortisol-RIA-CT, DIASource ImmunoAssays S.A., Louvain-la-Neuve, Belgium). All samples were thawed and centrifuged. Consecutively, 200 µl of each saliva sample was incubated with 500 µl of 125Iodine-labelled cortisol in tubes coated with anti-cortisol monoclonal antibodies. After 180 minutes of incubation in a water bath at 37°C, the content of each tube was decanted. Then all tubes were washed with 3 ml of wash solution and aspirated again. The same procedure was done for 7 calibrators. All tubes were counted in a gamma counter for 60 seconds. The bound radioactivity of calibrators was used for the construction of the calibration curve. The cortisol concentrations of the samples were determined by dose interpolation from the calibration curve. The limit of detection was 0.53 ng/ml. The obtained results of SC were compared with the reverence intervals proposed by Ivars et al. [2]. Normal values were defined as concentrations between 25th and 75th percentile, which corresponded to results between 1.02 and 2.97 ng/ml. The mean morning SC was calculated from repeated measures.
Statistical analysis
Results were presented based on the parameters of descriptive statistics, including numbers with percentages for categorical variables, mean values and standard deviations (SD), or median values with first and third quartile (Q1, Q3) for continuous variables. The Shapiro-Wilk test was applied to analyse the distribution of continuous variables. The groups were compared using the χ2 test, Fisher exact test, one-way ANOVA, and Kruskal-Wallis test. The Spearman rank test was used to analyse the correlation between the number of performed procedures and values of morning SC. Girls and boys were compared with the Mann-Whitney U test. The analysis was performed using Statistica software, version 13.3 (TIBCO Software Inc., Palo Alto, CA, USA). Statistical significance was set at a p-value of < 0.05.
Results
Study group
Initially, 71 newborns were eligible for the study. Out of them, 14 neonates were excluded from the analysis because 2 independent saliva samples were not obtained (Figure 1). In total, the study group consisted of 57 patients: 21 term, 17 moderate to late preterm, and 19 very preterm neonates with median GA of 40, 33, and 28 weeks, as appropriate. 33.33% of newborns from the moderate to late preterm group and 21.05% from the very preterm group received complete antenatal corticosteroid prophylaxis according to the guidelines of the American College of Obstetricians and Gynecologists [9]. The detailed characteristics of assessed patients are presented in Table I.
Table I
Characteristics of the study group
* Significant difference for paired comparison between term and very preterm neonates (p = 0.03) and between moderate to late preterm and very preterm neonates (p = 0.01).
** Significant difference for paired comparison between term and very preterm neonates (p = 0.006) and between moderate to late preterm and very preterm neonates (p = 0.02).
*** Significant difference for paired comparison between term and moderate to late preterm neonates (p = 0.002) and between term and very preterm neonates (p < 0.001).
Procedural pain during hospitalization
The mean duration of the hospital stay before saliva sampling was 6 ±4 days for term, 16 ±8 days for moderately to late preterm, and 53 ±21 days for very preterm newborns. The analysed groups differed in the median time of both mechanical ventilation (p < 0.001) and non-invasive respiratory support (p = 0.01, Table I). During the hospital stay, very preterm infants were exposed to 24 (12–38) skin-breaking procedures, whereas the exposure in moderate to late preterm and term neonates was significantly lower (6 [4–13] and 7 [3–11], as appropriate, p < 0.001). Similarly, very preterm infants experienced the highest number of total procedures related to hospitalization in comparison to the other groups (64 [43–100] vs. 24 [18–32] vs. 19 [15–26], p < 0.001).
Administration of corticosteroids
At the time of saliva sampling the patients did not receive corticosteroids. However, 21 (36.84%) infants had a history of treatment with systemic corticosteroids, whereas administration of local corticosteroids (inhaled, topical, or ophthalmic) was found in 11 (19.3%) patients. Both types of corticotherapy were used significantly more often in very preterm newborns in comparison to other groups (p < 0.001 for systemic and p = 0.001 for local corticosteroids).
Morning salivary cortisol
In 67% of patients, 3 independent saliva samples for SC assessment were obtained. In the remaining 33% of newborns, SC was measured twice. The groups did not differ in the interval between sample collections counted in days (p > 0.05). At the time of saliva sampling, 33 (57.89%) infants were fed orally, 20 (35.09%) obtained oral and nasogastric tube feeding, 2 (3.51%) were fed only enterally using the nasogastric tube, and 2 (3.51%) received partial enteral and parenteral nutrition.
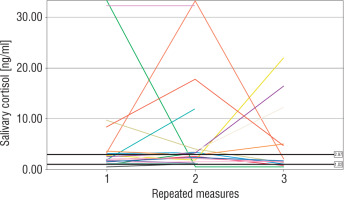
The number of measurements within the reference interval proposed by Ivars et al. was 29 (46%) for term newborns, 24 (47%) for moderate to late preterm infants, and 26 (46%) for very preterm infants (p > 0.05). The widest range of SC results was observed in very preterm neonates. In 4 (19%) term, 3 (18%) moderate to late preterm, and 4 (21%) very preterm infants all individual SC concentrations were within the proposed normal range. The results of repeated SC measurements are shown in Figures 2–4.
Very preterm newborns obtained higher mean morning SC in comparison to moderate to late preterm and term neonates; however, the difference between the groups was not significant (3.83 [1.67–8.81] ng/ml vs. 2.44 [1.94–4.38] ng/ml vs. 2.15 [1.5–5.25] ng/ml, p = 0.45). In term newborns the mean morning SC was negatively correlated with the number of invasive blood samplings (Rs = –0.44, p < 0.05). No correlation between the total number of experienced procedures or the number of skin-breaking procedures and the mean morning SC was found in any group (p > 0.05). However, we observed a significant relationship between the time of non-invasive ventilatory support and the mean morning SC in very preterm infants (Rs = –0.68, p < 0.05). Antenatal corticosteroid prophylaxis did not affect morning SC in preterm infants. Furthermore, we did not find any significant influence of either systemic or local corticosteroids on the mean morning SC in newborns (p > 0.05). No relationship between morning SC and other perinatal factors was observed. Girls were characterized with significantly lower morning SC than boys (1.97 [1.59–4.94] ng/ml vs. 3.66 [2.34–5.73] ng/ml, p = 0.03).
Discussion
To the best of our knowledge, this is the first study to examine the influence of repeated procedural pain related to hospitalization on HPA axis activity in preterm and term newborns. We performed a comprehensive analysis of not only skin-breaking but also other stressful procedures frequently performed in the ICU. In the present study, we observed a negative correlation between the number of invasive blood samplings and mean morning SC only in term newborns. In contrast, Ivars et al. did not find any significant relationship between the number of self-reported traumas and SC concentrations in one-month-old infants [2]. There was no relationship between exposure to procedural pain and mean morning SC in premature neonates, irrespective of the degree of prematurity. In contrast, Gunner et al. showed that greater pain-related neonatal stress was associated with higher basal SC levels in extremely preterm infants at 8 months of CA [8]. Another study revealed a gradual decrease of basal SC in preterm infants during the first 2 weeks of life [10]. Nevertheless, the direct influence of postnatal stress on the dampened SC levels was not analysed.
Importantly, the results of SC in term and preterm newborns with eventful perinatal history should not be referred to normal ranges for healthy term neonates. We observed that about 46% of all results were within the proposed reference range. Furthermore, we suggest that due to the high individual variability of SC concentrations, the assessment of HPA axis activity in newborns should never be based on a single value, but rather on a series of repeated measurements.
It seems that very preterm infants over 35 weeks of PMA obtain higher levels of morning SC than term and moderate to late preterm newborns. The data regarding morning SC in preterm newborns at age around DED are limited. In comparison to our results, Ivar et al. reported lower levels of morning SC in preterm neonates in similar PMA [5]. However, the analysed cohort was characterized by higher Apgar scores and more frequent use of antenatal corticosteroids than our study group. This leads to the conclusion that preterm newborns with higher illness severity seem to have higher morning SC levels.
The influence of antenatal corticosteroid prophylaxis on HPA axis activity has been comprehensively analysed, but the available studies presented inconsistent results. Karsli et al. revealed that antenatal corticosteroids affected only the level of 17-hydroxyprogestrone (17-OHP) in neonatal cord blood, whereas other studies showed significant reductions in both serum 17-OHP and cortisol levels in newborns [11, 12]. Although antenatal corticosteroids can potentially dampen the SC levels until 33 weeks of PMA, this effect is no longer observed at 2 and 3 months of CA [13–15]. In the present study, we did not observe any long-term influence of antenatal corticosteroid prophylaxis on morning SC levels in preterm infants.
The current data regarding sex differences in basal SC levels are ambiguous. Gunnar et al. observed lower basal SC in girls than in boys at different time points of infancy [16]. However, a review on gender-specific differences in HPA axis activity did not report significant differences in SC among children < 8 years old in 4 out of 5 included studies [17]. Male gender is a well-known risk factor for some of the complications of prematurity; hence, we postulate that the observed differences in morning SC are potentially caused by the illness severity, not the newborn’s gender [18].
The main strength of our study is its comprehensive prospective analysis of procedural pain related to hospitalization. It enabled the evaluation of total stress exposure related to ICU stay in preterm and term infants. The main limitation is the small sample size. However, the analysis of the power of the study revealed that based on the recruited groups (n = 19) the detectable difference in medians should be 2.45 ng/ml with the assumption of a significance level of 0.05. So, if the difference between the groups exists, it seems to be lower than 2.45 ng/ml, which can be considered clinically insignificant. The other limitation is that saliva sampling was performed only once per day. Indeed, multiple diurnal assessments of SC give significantly more insight into the functioning of the HPA axis. In addition, in 33% of assessed newborns, morning SC was calculated using only 2 independent saliva samplings. Because newborns are characterized by a wide range of SC concentrations, it could result in bias during calculation of the mean [19].
Conclusions
Although hospitalization in the ICU is related to high exposure to painful procedures, it seems that repeated stress does not deteriorate the morning HPA axis activity in preterm infants. However, it can potentially dampen the SC levels in term newborns. We suggest that the assessment of HPA axis activity in infants should be based on repeated measurements, due to the high individual variability of morning SC levels. Results of SC in infants with eventful perinatal history should not be referred to normal ranges for healthy term neonates. During the interpretation of SC results, gender-specific differences should also be considered.

 POLSKI
POLSKI