Introduction
Infectious mononucleosis (IM) is considered a benign lymphoproliferative disorder caused by the Epstein-Barr virus (EBV, human gamma-herpesvirus 4 – HHV-4). EBV is widespread in all human societies and cultures (humans are the only known reservoir of this pathogen), and approximately 95% of the world’s population has detectable IgG antibodies against EBV [1–3]. Infection occurs through close contact with an EBV-shedder: by sharing food or utensils, kissing, coughing, and sneezing (contact with saliva) or sexual intercourse, as well as after blood transfusions or organ transplantations [2]. The risk of infection is estimated at 10% [4]. About 10-20% of infected people develop full-blown IM preceded by a 30-50-day incubation period [2]. In the remaining infected persons (usually young children), the condition is mild or asymptomatic [2, 5]. Primary infection results in a life-long latency of EBV in memory B cells. The virus can periodically (during reactivation) spread in saliva throughout the life of an infected person [3, 6, 7]. Contagiousness lasts up to 6 to 18 months from the onset of the disease.
The classic triad of IM symptoms includes fever, pharyngitis, and lymphadenopathy. Patients may present with other signs and symptoms, such as fatigue, nausea, vomiting, anorexia, rash, hepatosplenomegaly, and abdominal pain. The condition is usually self-limiting and resolves within 2 to 3 weeks. Eighty to ninety percent of patients with primary EBV infection develop hepatitis with mild to moderate elevation of liver aminotransferases [5]. Liver enzyme values usually return to normal within a few weeks [8]. Symptoms of cholestasis may appear. EBV infection is a potential cause of acalculous cholecystitis as well [9]. Isolated cases of fatal liver failure in immunocompetent patients have been reported in the available literature [10].
The pathomechanism of parenchymal liver injury and cholestasis in primary EBV infection remains unclear. An important role may be played by EBV-infected CD8+ T cells in the liver, which secrete inflammatory mediators such as tumor necrosis factor α, interferon γ, and Fas ligand with a potential impact on the sinusoidal and tubular bile transport systems [11, 12]. EBV infection is not localized in hepatocytes and biliary cells.
Treatment of IM is symptomatic and includes adequate hydration and management of fever and pain with nonsteroidal anti-inflammatory drugs or acetaminophen. Patients who develop dehydration or complications such as difficulty breathing due to swelling of the pharyngeal lymphatic tissue or more severe hepatitis may require hospitalization.
To date, no target antiviral therapy has been developed. Acyclovir is not recommended – a meta-analysis of five randomized controlled trials involving 339 patients showed no significant clinical benefit [13].
Steroids have been ordered for their anti-inflammatory effect since the 1950s, but there are no clear guidelines for their use in IM. The above action of glucocorticoids results from the direct and indirect modification of the expression of genes encoding pro-inflammatory factors. According to a literature review, no clinical benefit from the use of steroids in IM was found in 8/10 trials. Two studies have shown the benefit of steroid therapy over placebo in reducing sore throat at 12 hours, but this effect was not maintained [4].Literature data indicate that corticosteroids may be given especially to patients who develop complications such as significant pharyngeal edema that threatens respiratory compromise, or autoimmune disorders: anemia, and thrombocytopenia [14]. On the other hand, the value of such treatment is controversial and steroids may delay clearance of the viral load [3].
Our study aimed to determine the differences in the course of hepatitis associated with primary EBV infection between the patients treated with steroids and those not receiving such therapy.
Material and methods
Patients
The study was based on a retrospective analysis of the medical records collected among patients aged 0-18 years hospitalized for IM due to primary EBV infection at the Department of Children’s Infectious Diseases, Medical University of Warsaw, Regional Hospital of Infectious Diseases in Warsaw, between August 2017 and March 2023.
The onset of IM was considered to be the appearance of the first signs and symptoms such as swollen cervical lymph nodes, sore throat, fever, gastrointestinal complaints, or jaundice. The diagnosis was confirmed by a positive result of a serological test detecting immunoglobulin M antibody to EBV viral capsid antigen – anti-EBV VCA IgM (chemiluminescence immunoassay – CLIA test system, LIAISON EBV IgM, DiaSorin S.p.A., Saluggia, Italy) – as a marker of acute infection.
Hepatitis associated with primary EBV infection was diagnosed based on the elevation of serum alanine aminotransferase (ALT) activity over age reference values [15]. In addition, the following laboratory tests were performed: serum aspartate aminotransferase (AST) and γ-glutamyl transferase (γ-GT) activity, and total serum bilirubin concentration with fractions. The main indication for determining the activity of γ-GT and total serum bilirubin concentration with fractions was symptoms of cholestasis: jaundice, pruritus, acholic stools, and dark urine. We also performed full blood count with differential and C reactive protein (CRP). Leucocytosis was defined according to age and sex standards [16].
A more severe course of the disease, especially with symptoms of cholestasis or markedly enlarged spleen and/or liver on palpation, was an indication for abdominal ultrasound. Splenomegaly was defined as the longitudinal dimension of the spleen exceeding the 97.5th percentile for age [17].
We divided the study population – children and adolescents diagnosed with primary EBV hepatitis – into two groups: the first cohort included patients who received steroids, and the second consisted of patients not treated with steroids.
The most common indication for steroid therapy was significant airway obturation with sleep apnea or allergic-toxic rash after amoxicillin.
We established the following exclusion criteria: 1. Coexisting cytomegalovirus (CMV) infection (positive CMV IgM), 2. Pre-existing liver diseases of any etiology, 3. Diagnosis of other viral liver infections: hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), 4. Prior pharmacotherapy with potential hepatotoxicity.
Statistical analysis
Statistical analysis of collected medical records was performed using STATISTICA 13.3 (StatSoft, Kraków, Poland). Data were presented as mean (standard deviation; SD) unless otherwise indicated. To compare clinical features between study groups, the χ2 test (for categorical variables) was performed; in the case of an insufficient number of observations in the subgroups, Fisher’s exact test was used. We analyzed the differences in laboratory findings, the duration of signs and symptoms, and the length of hospital stay (continuous variables) between the study groups using the Mann-Whitney U test. A p-value of < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
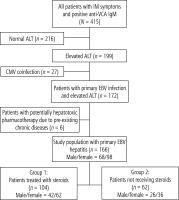
The medical records of 415 children and adolescents hospitalized due to IM associated with EBV infection within 79 months were retrospectively analyzed. In this cohort, 199 (47.9%) patients were diagnosed with hepatitis with increased ALT activity. Of this group, 33 (16.6%) were excluded from the study because of positive serological test results (IgM antibodies) for CMV (n = 27), and potentially hepatotoxic pharmacotherapy due to pre-existing chronic disease (n = 6). For the study, 166 patients, aged 18 months to 18 years (mean, 11.9 ±5.1 years), were recruited. In the first group of 104 patients (62.6%) who were treated with steroids the male-to-female ratio was 1 : 1.47. In the second cohort of 62 (37.3%) children who did not receive steroids the male-to-female ratio was 1 : 1.38.
Our patients received steroids intravenously, temporarily, mainly in the case of symptoms of upper respiratory tract obturation. Forty-eight children (46.1%) received dexamethasone, 47 (45.2%) hydrocortisone, and prednisolone were administered much less often – only in 8 patients (7.7%). Children up to 5 years of age were treated with hydrocortisone at a dose of 8-10 mg/kg/day, and older children at a dose of 4-8 mg/kg/day, in 3 divided daily doses. The dose of dexamethasone was 0.15-0.6 mg/kg/day in 4 divided daily doses. Prednisolone was administered at a dose of 1-4 mg/kg/day, in 3 divided daily doses. During hospitalization, patients received an average of 4.06 ±2.81 doses of steroids (from 1 to 14 doses). Most frequently, patients were administered steroids in the first and second weeks of illness.
The flowchart (Fig. 1) shows the enrolment of patients in the study.
Fig. 1
Flowchart showing recruitment of patients to the study. IM – infectious mononucleosis, ALT – alanine aminotransferase, CMV – cytomegalovirus, EBV – Epstein-Barr virus
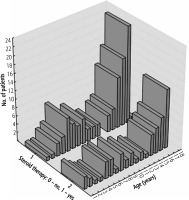
The age distribution in the two groups of the study population is shown in Figure 2.
Clinical course and complications
Analyzing the clinical course of IM in Groups 1 and 2, we obtained significant differences regarding the length of hospitalization (5.0 ±2.1 vs. 4.3 ±2.3 days; p = 0.015), the occurrence of the classic triad of IM symptoms (which was observed in 84.6% of children treated with steroids and in 72.6% of patients without steroid therapy; p = 0.000), fever – the most common clinical feature (90.4% of patients in Group 1 vs. 79.0% in Group 2; p = 0.040), and its duration (patients received steroids presented with fever for 7.5 ±4.7 vs. 5.9 ±4.8 days in children without steroid therapy; p = 0.049). Significant differences were observed in the occurrence of hepatomegaly (85.5% of children in Group 1 vs. 72.6% in Group 2; p = 0.040), and splenomegaly (73.1% vs. 56.4%; p = 0.027) as well. As regards age, the male-to-female ratio, the duration of signs and symptoms of IM, the incidence of abdominal pain and abdominal tenderness on palpation, and symptoms of cholestasis (jaundice, pruritus, dark urine, and acholic stools), they showed no statistically significant differences between the groups of patients. The differences in clinical features between the study groups are shown in Table 1.
Table 1
Differences in clinical features of infectious mononucleosis (IM) between study groups
Sixty-five patients (62.5%) treated with steroids and 15 (24.2%) children who had not received steroids developed complications of IM (p = 0.000). Obturation of the upper respiratory tract as the main reason for steroid administration was obviously more frequently observed among patients in the first group – 58 (55.7%) – than in the second group – 11 (17.7%); p = 0.000. Other common complications occurred without a statistically significant difference: allergic-toxic rash after amoxicillin administration was reported in 21 (20.2%) children in Group 1, and 7 (11.3%) in Group 2; thrombocytopenia with or without purpura was observed in 4 (3.8%) patients only in patients treated with steroids.
Other complications were observed rarely, and are presented in Table 2.
Table 2
Incidence of particular complications (other than the liver, biliary tract, or gallbladder pathology) in Groups 1 and 2
Laboratory and imaging test results
The mean number of white blood cells (WBC) assessed in the peripheral blood count (performed in all patients) in both groups of patients did not differ and was 14.75 ±5.76 cells/µl in the first group vs 14.39 ±8.49 cells/µl in the second group. Leukocytosis was found in 85 (81.7%) patients treated with steroids and 44 (70.9%) children not receiving steroids (p = 0.107). A white blood cell smear was performed in 162 (97.6%) patients and statistically significant differences were obtained in the percentage of neutrophils (33.5 ±12.2% in Group 1 vs. 19.4 ±14.5% in Group 2; p = 0.020). CRP concentration – assessed in 161 (96.9%) patients – also differed statistically significantly: 25.51 ±17.92 mg/dl vs. 15.46 ±11.11 mg/dl, respectively in the first and the second group; p = 0.000. There were no significant differences in the percentage of atypical lymphocytes, platelet count, and serum concentration of procalcitonin (PCT) and lactate dehydrogenase (LDH) in the study groups. Table 3 shows the comparison of laboratory data between study groups.
Table 3
Comparison of laboratory data between study groups
| Variable | Group 1 (n = 104) Mean ±SD | Group 2 (n = 62) Mean ±SD | P-value |
|---|---|---|---|
| WBC (cells/µl) | 14.75 ±5.76 | 14.39 ±8.49 | 0.101 |
| Neutrophils (%)a | 33.5 ±12.2 | 19.4 ±14.5 | 0.020 |
| Lymphocytes (%)a | 66.1 ±12.8 | 70.7 ±14.4 | 0.013 |
| Atypical lymphocytes (%)a | 25.5 ±14.3 | 25.8 ±14.9 | 0.801 |
| Platelets (cells/µl)b | 208.70 ±63.72 | 211.73 ±72.19 | 1.000 |
| CRP (mg/dl)c | 25.51 ±17.92 | 15.46 ±11.11 | 0.000 |
| PCT (ng/ml)d | 2.74 ±8.86 | 0.06 ±0.55 | 0.706 |
| LDH (U/l)e | 613.5 ±185.66 | 735.92 ±254.35 | 0.231 |
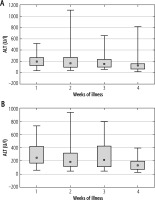
The analysis of ALT activity in both study groups in the successive weeks of the IM showed that in the first, second, and third weeks, ALT levels were higher in Group 2, and the highest values were observed in the first and third weeks of the disease. In the first group, the ALT level was the highest in the first week of IM, and a downward trend was observed in the following weeks, without a peak in the third week as in Group 2. Figure 3 shows the mean activity of ALT in successive weeks of EBV hepatitis in both groups of patients.
Fig. 3
Mean activity of alanine-aminotransferase (ALT) (± standard deviation) in successive weeks of Epstein-Barr virus (EBV) hepatitis in patients treated with steroids (Group 1), p = 0.129 (A), and in patients not treated with steroids (Group 2), p = 0.277 (B). The Kruskal-Wallis test was used for changes over time
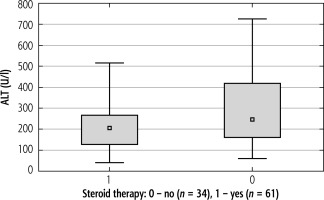
The medians of serum ALT activity in the study groups differed statistically significantly only in the first week of the disease, and their mean values were 205.34 ±115.40 IU/l (ranging from 39.00 to 516.00) vs. 288.82 ±170.16 IU/l (ranging from 50.00 to 725.00) for Groups 1 and 2, respectively; p = 0.024. ALT levels in the first week of IM were evaluated in 95 (57.2%) patients: 61 (58.6%) from the first group and 34 (54.8%) from the second group. The comparison of the median activity of ALT in both groups in the first week of the disease is shown in Figure 4.
Fig. 4
Comparison of median activity of alanine aminotransferase (ALT) in the 1st week of infectious mononucleosis (IM) in Groups 1 and 2; p = 0.024 in the Mann-Whitney U test (n = 95)
The median ALT serum concentrations in the compared groups of patients in the successive weeks (from 2nd to 4th) of the disease did not differ statistically significantly. Mean ALT values assessed from the first to fourth week of IM in both study groups are shown in Table 4.
Table 4
Values of alanine aminotransferase (ALT) activity in Groups 1 and 2 in successive weeks of the disease
Aspartate aminotransferase activity in the first three weeks of the disease was higher among patients not treated with steroids than in the patients undergoing this therapy. In both groups, slightly higher AST levels can be observed in the first and third weeks of IM. However, these differences are statistically significant only in children not receiving steroids (p = 0.024). Figure 5 shows the mean activity of AST in successive weeks of EBV hepatitis in both groups of patients.
Fig. 5
Mean activity of aspartate-aminotransferase (AST) (± standard deviation) in successive weeks of Epstein-Barr virus (EBV) hepatitis in patients treated with steroids (Group 1), p = 0.256 (A), and in patients not treated with steroids (Group 2), p = 0.024 (B). The Kruskal-Wallis test was used for changes over time
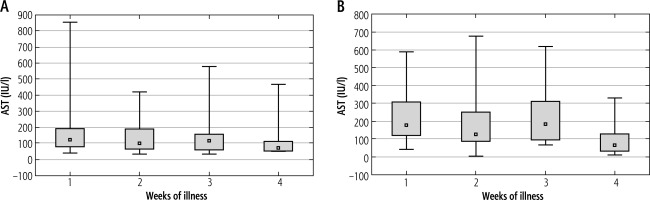
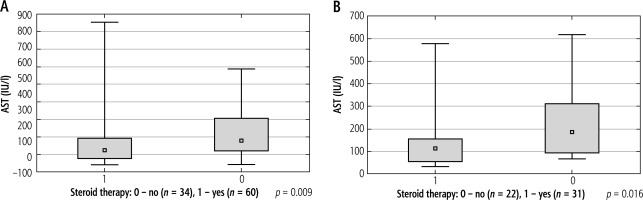
The median AST levels in the study groups differed statistically significantly in the first and third weeks of IM. The mean values of AST activity in the first week were: 170.63 ±159.47 IU/l (ranging from 40.00 to 852.00) vs. 218.85 ±128.22 IU/l (ranging from 42.00 to 589.00) for Groups 1 and 2, respectively; p = 0.009. The mean AST levels in the third week were: 151.09 ±138.57 IU/l (ranging from 31.00 to 578.00) vs. 235.50 ±170.27 IU/l (ranging from 67.00 to 617.00) for Groups 1 and 2, respectively; p = 0.016. AST activity in the first and third weeks of the disease was evaluated in 94 (56.6%) patients and 53 (31.9%) children, respectively. The comparisons of the median activity of AST in both groups in the first and third weeks of the disease are shown in Figure 6.
Fig. 6
Comparison of median activity of aspartate aminotransferase (AST) in Group 1 (p = 0.009) and Group 2 (p = 0.016) in the A) 1st (n = 94) and B) 3rd weeks (n = 53) of infectious mononucleosis (IM) in the Mann-Whitney U test
The median AST serum activity in the compared groups of children in the second and fourth weeks of the disease did not differ statistically significantly. Mean AST values evaluated from the first to fourth week of IM are presented in Table 5.
Table 5
Values of aspartate aminotransferase (AST) activity in Groups 1 and 2 in successive weeks of the disease
Among patients not receiving steroids, a peak of γ-GT activity was observed in the second week of IM. In the group of children treated with steroids, γ-GT levels showed an increasing trend with the duration of the disease. However, the differences in γ-GT levels in the successive weeks of the disease in both groups of patients were not statistically significant. Figure 7 shows the mean activity of γ-GT in successive weeks of IM in both cohorts of patients.
Fig. 7
Mean activity of γ-glutamyl transferase (γ-GT) (± standard deviation) in successive weeks of Epstein-Barr virus (EBV) hepatitis in patients treated with steroids (Group 1), p = 0.697 (A), and in patients not treated with steroids (Group 2), p = 0.053 (B). The Kruskal-Wallis test was used for changes over time
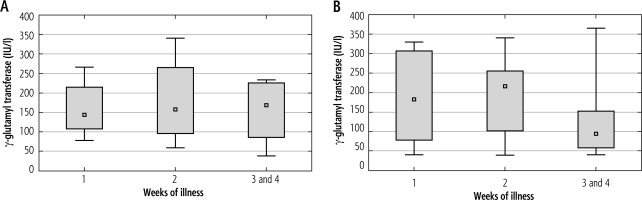
The median γ-GT levels between the study groups did not differ statistically significantly in any of the weeks of illness. Mean γ-GT values in successive weeks of the disease are shown in Table 6.
Table 6
Values of γ-glutamyl transferase (γ-GT) activity in Groups 1 and 2 in successive weeks of the disease
As described for γ-GT activity, total serum bilirubin concentration in children not treated with steroids was highest in the second week of the illness. However, these differences were not statistically significant. Among patients receiving steroids, total bilirubin remained at a comparable level throughout the course of the disease. Figure 8 shows the mean total serum bilirubin concentration in successive weeks of disease in both groups of patients.
Fig. 8
Mean total serum bilirubin concentration (± standard deviation) in successive weeks of Epstein-Barr virus (EBV) hepatitis in patients treated with steroids (Group 1), p = 0.834 (A), and in patients not treated with steroids (Group 2), p = 0.424 (B). The Kruskal-Wallis test was used for changes over time
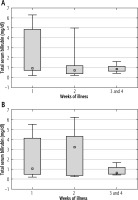
There were no significant differences between total serum bilirubin concentrations between both study groups in the successive weeks of IM. Table 7 contains the mean values of total serum bilirubin concentrations in both groups, determined in the successive weeks of the disease.
Table 7
Values of total bilirubin serum concentration in Groups 1 and 2 in successive weeks of the disease
Patients receiving steroids had a mean direct bilirubin serum concentration of 1.46 ±2.08 mg/dl (from 0.04 to 5.10). Among them, 8 (7.7%) had direct bilirubin at least 20% of total serum bilirubin concentration. The mean direct bilirubin in Group 2 (steroid-naïve) was 1.70 ±1.98 mg/dl (from 0.08 to 5.38). In 10 (16.1%) children of this group direct bilirubin accounted for at least 20% of total bilirubin.
Abdominal ultrasonography was ordered more often in patients not treated with steroids – 80.7% vs. 62.5% in Group 1 – but abnormal results were obtained in both groups in similar percentages of patients (97.2% in Group 1 vs. 95.2% in Group 2). The incidence of hepatomegaly, splenomegaly, and periportal lymphadenopathy was similar in both groups, as well. Children treated with steroids more often presented with perisplenic, intraperitoneal, and periportal fluid collection. However, gallbladder wall thickening was observed with higher frequency in patients not treated with steroids (14.5% vs. 7.7% in Group 1). The percentage of patients with particular abnormalities in the abdominal ultrasound examination in both groups is shown in Figure 9 and Table 8. Differences in the incidence of the above-described abnormalities were not statistically significant.
Fig. 9
Abnormalities in abdominal ultrasound in patients receiving steroids and not treated with steroids
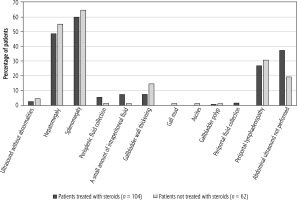
Table 8
Incidence of particular abnormalities in abdominal ultrasound examination in the studied groups of patients
Treatment
The most common indications for hospitalization of children with IM are high or long-lasting fever, dehydration, and symptoms of upper respiratory tract obturation with sleep apnea. Therefore, symptomatic treatment, including the administration of antipyretics and adequate hydration, is the primary management.
The number of patients who received empirical antibiotic therapy before admission to the hospital and before the diagnosis of IM was significantly higher in Group 1 than in Group 2, and was respectively 68 (65.4%) and 23 (37.1%); p = 0.000. The main reason for initiating antimicrobial treatment was pharyngitis. The most commonly prescribed antibiotics were: cefuroxime axetil, amoxicillin with clavulanic acid, phenoxymethylpenicillin, azithromycin, and amoxicillin. In Group 1, antimicrobial therapy was continued after hospital admission in 43 (41.3%) patients, and in 16 (15.4%)children who had not previously been treated with antibiotics, it was started. In 12 (19.3%) patients in Group 2 antibiotic therapy was continued during hospitalization. In this group, antimicrobial treatment was started after admission to the hospital in 14 (22.6%) children. The most commonly used substance during hospitalization was cefuroxime. Eighteen patients (17.3%) in the first group and 8 (12.9%) in the second group were treated with more than one antibiotic (p = 0.435).
Discussion
Our retrospective study is an attempt to characterize two groups of patients hospitalized with hepatitis caused by primary EBV infection. The first group includes patients treated with steroids due to complications of infectious mononucleosis (mainly upper respiratory tract obturation), and the second group consists of children not receiving the above therapy. In both groups, there is a noticeable predominance of persons in preschool and early school age (4-8 years old) and adolescents (14-18 years old), which is characteristic of the epidemiology of IM [18–20]. However, the above-described distribution of age groups is more pronounced among patients treated with steroids. Both groups are also characterized by a similar gender distribution: the percentage of males was 40.1% and 41.9%, respectively, in Groups 1 and 2.
The rich symptomatology of IM causes patients to present with a diverse constellation of symptoms and possible complications [21, 22]. There were also some differences between the two groups we studied. When analyzing the course of the disease, we found that patients in the steroid-treated group stayed longer in the hospital, and more often presented the classic features of IM with fever, which was the most common clinical symptom in them and lasted longer than in Group 2. They were also more likely to have hepatosplenomegaly on palpation, although the presence of other clinical signs suggestive of hepatitis (with features of cholestasis in some cases) was not significantly different between the two groups. Complications other than upper airway obturation occurred with similar frequency in both cohorts.
As regards the laboratory test results other than those evaluating liver function, statistically significant differences occurred only in the percentage of neutrophils in the white blood cell smear and CRP concentration. Both of these parameters were higher in patients who received steroid therapy.
The available literature provides an analysis of the laboratory test results assessing liver function in the course of IM [8, 9, 23]. However, it is difficult to find such research in the context of steroid therapy used in these patients. Analysis of the course of ALT and AST activity during the disease showed higher activity of both enzymes in the first, second, and third weeks of IM in patients not treated with steroids. However, statistically significant differences were observed only in the first week for ALT and in the first and third for AST. In an analysis involving the variability of enzyme activity over time, levels of both enzymes showed peaks at the first and third weeks of the disease in steroid-naïve patients. However, statistically significant differences were seen only for AST activity. Our observations are consistent with the results of other available studies [23]. These results may indicate greater severity of hepatitis (defined as increased ALT and AST levels) in patients with less pronounced upper respiratory tract IM symptoms: pharyngitis and tonsillitis, cervical lymphadenopathy, and consequent upper airway obturation. Despite the observed lower activity of ALT and AST in the first three weeks of the disease in patients treated with steroids, in the fourth week (usually after the end of steroid therapy) the levels of both enzymes were higher than in patients not receiving steroids. Thus, the anti-inflammatory effect of temporarily administered glucocorticosteroids (in doses appropriate for the treatment of the complications of IM) might be insufficient to achieve a sustained reduction in hepatic inflammation. However, this conclusion would require confirmation in further (more detailed) research.
Cholestasis is a possible complication of EBV-associated hepatitis [24–26]. Concerning the results of laboratory tests useful in the diagnosis of cholestasis (γ-GT and total serum bilirubin concentration with fractions), no statistically significant differences were obtained between the study groups. γ-GT activity was higher in steroid-naïve patients in the first two weeks of illness. Among these patients, peak γ-GT levels were observed in the second week of IM, as was the case for total bilirubin. Direct bilirubin of at least 20% of total bilirubin was observed more frequently in steroid-naïve patients (16.1%) than in children receiving steroids (7.7%).
Abdominal ultrasonography revealed perisplenic, intraperitoneal, and periportal fluid collection more often in patients receiving steroids, but gallbladder wall thickening was described with higher frequency among steroid-naïve patients. However, the above differences did not show statistical significance.
Patients who required steroids significantly more often started empiric antibiotic therapy before admission to the hospital. They were also more likely to continue antimicrobial treatment during hospitalization. Available literature data also indicate frequent administration of antibiotics in patients with upper respiratory tract complications of IM [27–29]. However, in our patients, the above therapy was implemented only after admission to the hospital in a larger proportion of steroid-naïve patients.
An undoubted limitation of our study is its retrospective nature and the fact that not all patients in both study groups underwent all laboratory tests that would allow for a comprehensive assessment of liver function and abdominal ultrasound. However, our analysis concerns a fairly large cohort of patients. Literature data related to this subject are limited, and most studies to date have been conducted on much smaller groups [4, 30, 31]. It should be noted that the doses of corticosteroids used in our patients were adjusted to treat airway obturation. Perhaps administration of higher doses would result in a stronger anti-inflammatory effect in hepatitis.
In conclusion, our study suggests that the two analyzed groups may differ in the course of hepatitis in primary EBV infection, especially in the first weeks of IM, when the laboratory features of hepatitis were more pronounced in children not requiring treatment with steroids. However, there was a noticeable trend for higher values of liver parameters at the end of the disease (suggestive of delayed resolution of hepatitis) in patients receiving steroids, but without statistical significance. A possible explanation for the above phenomenon is potentially delayed viral clearance caused by steroid therapy [3]. Cessation of steroid treatment and the disappearance of their anti-inflammatory effect could increase the activity of liver enzymes.
Institutional Review Board Statement
Ethical review and approval were waived for this study because the study was retrospective, non-interventional, and based on data collected in the department’s database. Therefore, it does not require approval of the Ethics Committee. Due to the retrospective nature of the present study, written consent from participants was not necessary. The patients’ data were protected according to the European Union General Data Protection Regulation.