Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder that is distinguished by the inflammation and abnormal growth of synovial tissue within the joints, resulting in irreversible destruction of cartilage and bone, joint deformity, and serious complications [1]. Rheumatoid arthritis impacts around 1% of the global population, with a male-to-female ratio of approximately 1 : 3 [2, 3]. As current drug treatments for RA have limited efficacy, it is important to study and understand the disease mechanism. There is substantial evidence from various studies that supports the critical role of hypoxia-inducible factor-1 (HIF-1) in the development and progression of RA, and the hypoxia microenvironment in joints is the main inducer of HIF-1 production [4]. Many intricate signaling pathways and molecular pathological processes are involved in it. HIF-1 is the central regulator of all hypoxia adaptations. Inhibiting HIF-1 expression by improving joint hypoxia is a potential treatment target for RA. This paper reviews the structural characteristics, regulation, and research progress of HIF-1 in the pathogenesis of RA.
Structure and function of HIF-1
Structure of HIF-1
Hypoxia-inducible factor-1 is a heterodimeric transcription molecule which belongs to a group of different types of HIF which includes HIF-1, HIF-2 and HIF-3 [5]. The HIF-1 protein is made up of two subunits, HIF-1α and HIF-1β, with HIF-1α being the active and regulatory part, which is located in the q21-24 region of chromosome 14 [6]. The amount of oxygen present in cells strictly regulates the expression of HIF-1α. HIF-1β, also referred to as aryl hydrocarbon receptor nuclear translocator (ARNT) [7], remains consistently and stably expressed within the nucleus and is resistant to hypoxic conditions. Both subunits of HIF-1 have an N-terminus that consists of the PAS-A and PAS-B domains and the basic helix-loop-helix (bHLH) domain [4]. These structures form heterodimers with the two subunits of HIF-1, which are then recognized and bound to the DNA-binding site of HIF to form a nuclear dimer. HIF-1α regulates the magnitude of HIF-1 activity, and its hydrolysis under normal oxygen conditions is regulated by the oxygen-dependent degradation domain (ODDD) on HIF-1α. ODDD degrades the proteasome through ubiquitination of the N-transcriptional activation domain (N-TAD). The transcriptional activation domains at the C-terminus are called C-transcriptional activation domains (C-TADs), which play a fine-tuning role in transcription [8]. Once HIF-1α combines with HIF-1β to form a heterodimer, it eventually activates the transcription of HIF-1 target genes by binding to hypoxia response elements (HREs) [9].
Functions of HIF-1
Hypoxia-inducible factor-1 is an important transcription factor that responds to changes in oxygen concentration and can be induced by hypoxic environments. It is involved in various important biological reactions, such as converting the glucose metabolism of cells in a hypoxic environment from the tricarboxylic acid cycle (TCA) to glycolysis, due to which the cells are in a state of continuous differentiation in the state of hypoxia [10]. Furthermore, HIF-1 can stimulate angiogenesis by triggering the production of vascular endothelial growth factor (VEGF), which participates in angiogenesis and leads to many diseases [11]. HIF-1α also stabilizes chondrocytes, making them survive [4]. The functions of HIF-1 in RA are mainly in promoting inflammation, angiogenesis and cartilage destruction [4].
Regulation of HIF-1 expression
PHDs/HIF-1α/pVHL pathway
Prolyl hydroxylase domain proteins (PHDs) play a vital role in regulating HIF-1α and are mainly involved in HIF-1α degradation [8]. The family of PHDs consists of PHD-1, PHD-2, and PHD-3, with PHD-2 primarily targeting the N-TAD of HIF-1α [8]. Moreover, PHD-2 is the most critical enzyme for regulating the stability of HIF and its downstream target genes in rheumatoid arthritis synovial fibroblasts (RASF) [12]. A study conducted by Barbara Muz et al. showed that HIF-1α expression soared when PHD-2 was knocked out in human RA fibroblast-like synoviocytes (FLS) [13]. It is reasonable to believe that stabilization of PHD-2 expression may be a potential therapeutic approach towards RA. Under normoxic conditions, PHDs modify HIF-1α, requiring iron (Fe2+) as an enzyme cofactor, with α-ketoglutaric acid and oxygen as substrates [12]. Proline at two key positions (Pro564 and Pro402) on the HIF-1α protein is hydroxylated by PHDs. The modified HIF-1α can be recognized and bound by the tumor suppressor von Hippel-Lindau (vHL) and subsequently enter the ubiquitination process, which involves modification by the vHL degradation complex. Finally, HIF-1α is degraded through the 26S proteasome pathway[14]. The oxygen-rich environment can maintain the high activity of PHDs. However, under low oxygen conditions, the enzyme activity of PHDs decreases, making it difficult to complete the hydroxylation modification of HIF-1α, resulting in vHL being unable to recognize, bind, and degrade HIF-1α, leading to a significant buildup of HIF-1α within cells [15]. This accumulation can result in a series of physiological responses mediated by HIF-1α, which can lead to related diseases.
Factor-inhibiting HIF (FIH)
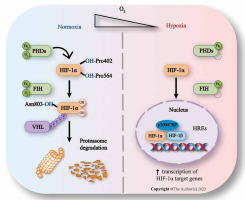
Factor-inhibiting HIF belongs to the same family of dioxygenases as PHDs, and it also serves a function in the modulation of HIF-1α [8]. Like PHDs, the enzymatic activity of FIH is directly regulated by oxygen, but it can modify HIF-1α under lower oxygen concentration (≥ 1%) [8]. Under normoxic conditions, the expression of FIH mRNA level is high and comparable to PHD-2 but the knockdown of FIH exerts no impact on the HIF level in RA-FLS [13]. Unlike PHDs, which act on N-TAD, FIH regulates the function of HIF by inactivating C-TAD, thus preventing the formation of the heterodimer and the transactivation of HIF in normal oxygen [8]. The C-TAD of HIF-1α contains asparagine residues (Asn803 in human HIF-1α) targeted by FIH hydroxylation [16]. FIH mediated hydroxylation of these residues obstructs the binding of the C-TAD of HIF-1α with cAMP-responsive element-binding protein and p300 protein (CBP/P300) via steric hindrance [17]. FIH-1 is capable of binding to both HIF-1α and pVHL, suggesting that the suppressive effect of FIH-1 on HIF-1α may result from the formation of a ternary complex involving FIH-1, HIF-1α, and pVHL [8]. Additionally, FIH can modulate HIF-1α through various mechanisms. Along with PHDs and FIH, acetylation of HIF-1α is also an essential regulatory mechanism [18]. The basic regulation of HIF-1 expression is summarized in Figure 1.
Fig. 1
Regulation of HIF-1 expression. In normal oxygen conditions, HIF-1α is modified by PHDs and recognized by VHL before being ubiquitinated by the proteasome pathway. FIH binds to HIF-1α and pVHL, preventing C-TAD from interacting with CBP/p300. In hypoxic environments, PHDs and FIH are inhibited, causing HIF-1α to accumulate and form heterodimers with HIF-1β. This recruits CBP/p300 and activates transcription of HIF-1 target genes by binding to HREs on promoters or enhancers
Regulation of inflammatory cytokines
Pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-6, and IL-8 in RASF are directly regulated by HIF-1α, which mediates interactions between T cells/B cells and RASF [19]. TNF-α is a crucial pro-inflammatory cytokine involved in the pathogenesis of RA. It contributes to the destruction of cartilage, the proliferation of RA cells, and the formation of pannus, leading to the progression of RA [20, 21]. Moreover, TNF-α regulates HIF activity through common intracellular signaling pathways, such as nuclear factor κB (NF-κB) and phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt) [22, 23].
Interleukin 1β, secreted by monocytes and macrophages, is a pro-inflammatory cytokine that induces the expression of HIF-1α protein in different cell types [22]. HIF-1α protein accumulation can be detected 4 h after the onset of IL-1β action, and is dependent on the concentration and exposure time of IL-1β [22]. Studies have shown that IL-1β expression differs in various cells, indicating that it has some cell specificity for the expression of HIF-1α mRNA [22]. Additionally, IL-1β can also stimulate the secretion of VEGF in various cells, promoting angiogenesis and contributing to the development of RA [24].
Regulation of other related signal pathways
Notch pathway
The Notch signaling pathway is a well-conserved mechanism of communication between cells, which plays a crucial role in coupling of bone formation with angiogenesis and osteogenesis [25]. The Notch signaling pathway in mammals involves four different receptors, namely Notch1-4 [26]. Direct interaction between HIF-1α and Notch intracellular domain (NICD) can occur. HIF-1α is recruited onto the transcriptional complex of NICD and binds to the corresponding promoter of Notch to activate the Notch target gene [27]. The expression of NICD is negatively correlated with oxygen levels in synovial tissue, and under hypoxic conditions, the invasion and angiogenesis in RA can be induced by the interaction between Notch1 and HIF-1α [28]. Blocking the Notch signaling pathway could potentially offer a novel therapeutic approach for RA by disrupting the biological functions of the HIF-1α protein [28].
PI3K/AKT pathway
The PI3K/Akt signaling pathway is responsible for regulating a wide range of cellular activities [29]. PI3K, which belongs to the lipid kinase family, can phosphorylate inositol ring 3'-OH groups in plasma membranes [30]. Akt is considered to be the central mediator of the PI3K/Akt signaling pathway, and it can phosphorylate several essential downstream targets [31]. This pathway is frequently associated with HIF-1α, as the stability of HIF-1α is linked to the PI3K/Akt signaling pathway under hypoxic conditions [32]. PI3K can activate mammalian target of rapamycin (mTOR) through phosphorylation of AKT, leading to upregulation of HIF-1α expression [33]. Recent research has revealed that Wu-Tou decoction (WTD), a traditional Chinese medicine, can block the PI3K-AKT pathway and reduce the expression of HIF-1α, providing a promising therapeutic strategy for RA [33].
NF-κB pathway
Nuclear factor κB is a group of transcription activators that are often activated during the early stages of cellular hypoxia and have a close relationship with the transition of HIF-1α from partial to full activation [18]. Under normoxic conditions, the activity of inhibitor of NF-κB (IKB) kinase (IKK) is inhibited by PHD. However, hypoxia can decrease the activity of PHD, allowing IKK to phosphorylate IKB and activate the transcription of NF-κB dependent genes [12]. Additionally, under hypoxic conditions, activation of NF-κB can also occur through the PI3K/Akt pathway [18]. The overexpression of NF-κB in the synovial tissue of RA is implicated in the regulation of gene expression of TNF-α, IL-6, IL-8, VEGF, matrix metalloproteinase-1 (MMP-1), and MMP-3. Moreover, NF-κB works in conjunction with HIF-1 to contribute to the progression of RA [12]. In RA, proinflammatory M1 macrophages can activate the signaling cascade between NF-κB and HIF-1α, leading to the production of reactive oxygen species (ROS) and the amplification of M1 macrophage polarization, thereby worsening the progression of RA. Recent studies using an ROS-responsive artesunate prodrug nano-system that co-delivers dexamethasone have effectively inhibited this process and showed positive results in treating rat RA [34]. The general connections between other signaling pathways and HIF-1 are depicted in Figure 2.
Role of HIF-1 in rheumatoid arthritis
HIF-1 and hypoxic microenvironment
The hypoxic microenvironment within the synovial membrane of RA patients is crucial for the occurrence and development of RA lesions and it is a causative factor associated with various pathophysiological changes [4]. Prolonged hypoxia in the joints of RA patients results in increased HIF-1 expression in the synovial membrane. This leads to activation of key glycolytic enzymes, shifting cells from oxidative phosphorylation to anaerobic glycolysis as an adaptation to the hypoxic environment. Consequently, there is increased glucose uptake and glycolysis. In the hypoxic microenvironment, HIF-1α-dependent glycolysis provides energy for various inflammatory cells, thus initiating and sustaining the RA inflammatory response. Glycolysis also leads to acidosis, elevated levels of lactic acid, H+, and ketone bodies, further upregulating HIF-1α expression and intensifying the positive feedback loop [35] (Fig. 3A). Similarly, the proliferation of synovial cartilage and the formation of pannus can make diffusion in the synovial membrane difficult, aggravating the hypoxia in the synovium, and exacerbating the positive feedback response [36].
Fig. 3
The role of HIF-1 in rheumatoid arthritis. A) In rheumatoid arthritis, massive HIF-1α in the synovial membrane increases transcription of key enzymes for glycolysis, inhibiting pyruvate conversion to lactic acid and entering TCA. B) HIF-1α upregulates cytokines and chemokines, promoting synovial inflammation in rheumatoid arthritis. C) HIF-1α stimulates the production of VEGF and MMPs in FLS, resulting in angiogenesis and cartilage destruction
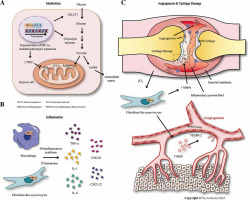
In the synovial tissue of RA, FLS are the predominant cells and play a vital role in the progression of RA. FLS have the capability to induce synovial inflammation by generating mediators that attract and activate inflammatory cells, leading to the formation of invasive pannus and the production of MMPs and cathepsins, causing tissue damage [37]. Under hypoxic conditions, FLS can produce CXCL12 and VEGF and allow HIF-1α to escape the influence of prolyl hydroxylase [38]. The increased expression of HIF-1α can further stimulate RA-FLS to produce IL-8 and IL-1 [38]. Under hypoxia, HIF-1α can regulate the expression of Notch1, which promotes FLS migration and epithelial-mesenchymal transition (EMT) [38]. LY411575 (an inhibitor of NICD cleavage) can effectively inhibit collagen-induced arthritis development in rats [38]. These mechanisms collectively contribute to the pathological progression of RA under a hypoxic microenvironment.
HIF-1 and inflammation
The inflammatory response associated with RA is heavily influenced by HIF-1, which can be regulated through multiple pathways. The upregulation of HIF-1α leads to increased expression of several cytokines including interferon γ (IFN-γ), IL-6, IL-8, IL-17, and IL-33 [39], thereby promoting the production of RA synovial inflammation. Recent studies have found that treatment of RA-FLS with TNF-α significantly increases the expression of IL-6, IL-8, and HIF-1α. TNF-α also enhances the phosphorylation of NF-κBp65 and promoter activity in RA-FLS [40]. These effects can be attenuated by the HIF-1α inhibitor lificiguat (YC-1) and the NF-κB inhibitor BAY11-7082 respectively [40]. Moreover, HIF-1α also plays a significant role in immune cells. For example, HIF-1α can perpetuate and sustain the expansion of RA-SLF-mediated inflammatory Th1 and Th17 cells, prolonging the interaction between RA-FLS, T cells, and B cells, leading to the production of inflammatory cells and autoantibodies [19]. Elevated levels of HIF-1α in M1 macrophages enhance the synthesis of inflammatory cytokines, while concurrently suppressing NF-κB activity to prevent excessive inflammation activation [4]. In rat model experiments with collagen-induced arthritis, HIF-1α was silenced by siRNA, resulting in the down-regulation of inflammatory cytokine expression, thus alleviating collagen-induced arthritis in rats [41]. The general mechanism is illustrated in Figure 3B.
HIF-1 and angiogenesis
Angiogenesis is a prevalent characteristic of RA lesions, and the regulation of VEGF gene expression by HIF-1α is a critical factor in its pathogenesis [42]. The interaction between VEGF and its receptor, VEGFR-2, which is located on the surface of vascular endothelial cells, is crucial for promoting endothelial cell proliferation, migration, vascular reconstruction, and changes in vascular permeability [43]. Toll-like receptor (TLR)-activated M1 macrophages have the capability to release pro-inflammatory cytokines, including IL-1, IL-6, IL-8, and TNF-α [44]. These released cytokines, in conjunction with the increased intracellular expression of HIF-1α triggered by hypoxia, contribute to the synthesis of pro-angiogenic factors such as VEGF and basic fibroblast growth factor in FLS, macrophages, and T cells [44]. Specifically, VEGF acts on VEGFR-2, promoting synovial vascular proliferation. This vascular proliferation further aggravates synovial hyperplasia and inflammation, exacerbating hypoxia and creating a vicious cycle [44]. Studies have indicated that in the collagen-induced model of RA mice, IL-35 has the potential to decrease the expression of VEGF and its receptors. This finding suggests that IL-35 might have a role in suppressing angiogenesis in this pathological mechanism [45]. Recent studies have also found that moxibustion can enhance the analgesic and anti-inflammatory effects of conventional drugs by downregulating HIF-1α/VEGF levels and inhibiting angiogenesis, thereby alleviating synovial lesions in RA patients [46].
HIF-1 and cartilage destruction
Cartilage destruction is a key feature of RA lesions. The survival of chondrocytes in a hypoxic environment is closely linked to HIF-1, which helps chondrocytes adapt to low oxygen concentration in the periphery [47]. MMPs, a group of enzymes that degrade extracellular matrix proteins, have the ability to increase the destruction of articular cartilage, and their activity is regulated by tissue inhibitors of metalloproteinases (TIMPs). Under hypoxic conditions, FLS upregulate the expression of MMP-1 and MMP-3, while the level of TIMP-1 decreases [12]. HIF-1 promotes the secretion of MMP-13 and MMP-1 mediated by IL-1β and the expression of MMP-9 and MMP-17 mediated by IL-2/TNF-α [48]. Meanwhile, HIF-1α can significantly increase the production of highly active MMPs by inducing the transcription factor Ets-1, leading to direct cartilage damage and destruction of articular cartilage [36]. Recent studies have found that the invasiveness and inflammation of RA-FLS can be regulated by the long noncoding RNA (lncRNA) LINK-A through HIF-1α. The expression of MMP-1, MMP-3, MMP-9, and MMP-13 induced by TNF-α can be reduced by si-LINK-A transfection, and the secretion levels of these MMPs are also decreased when HIF-1α is knocked out [49]. The results indicate that LINK-A could serve as a promising therapeutic target for the treatment of RA [49]. In addition, HIF-1α-dependent BCL2-binding protein 3 (BCL-2 interacting protein 3, or BNIP3) is activated, causing autophagy. Knocking out either HIF-1α or BNIP3 genes can significantly weaken the autophagy response and reduce the degree of cartilage destruction [50]. Furthermore, the regulation of HIF-1 is essential for maintaining specific markers of cartilage production (such as aggregating glycan and SOX9) and inhibiting cartilage hypertrophy [4]. Angiogenesis and cartilage destruction are illustrated in Figure 3C.
Clinical significance of HIF-1 in rheumatoid arthritis
In essence, HIF-1 plays a role in numerous aspects of RA initiation and progression, including inflammation, angiogenesis, and synovial hyperplasia [4]. Targeting HIF-1α inhibitory drugs is a promising approach for treating RA patients who have not responded to traditional drugs. Currently, there are several HIF inhibitors in development, including aminoflavone, which inhibits HIF transcription and translation, vorinostat, which reduces the stability of HIF, and acriflavine, which inhibits HIF heterodimerization; many of them have shown promise in clinical trials [51, 52]. However, most of the approved or used drugs are not specific for HIF subtypes, while specific HIF-1α inhibitors are currently under development. Some HIF inhibitors, such as CAP-based anti-inflammatory HIF-1α siRNA-encapsulating nanoparticle granules, have been used to treat RA and have shown great potential [53]. Furthermore, many HIF inhibitors have made significant progress in treating various cancers [54]. However, the side effects of HIF inhibitors should not be ignored. Currently available HIF inhibitors, such as echinomycin and YC-1, have dose-dependent side effect [52], such as gastrointestinal symptoms, elevated liver enzymes, and decreased platelet and granulocyte counts [55]. These side effects should be taken into consideration for future applications.
Conclusions
In summary, HIF-1 has emerged as an important transcription factor in the hypoxic microenvironment of RA joints. It plays a crucial role in the pathogenesis of RA, which is regulated by multiple mechanisms and signaling pathways. Understanding the regulatory modes and molecular mechanisms between HIF-1 and RA is crucial for the exploration of new potential therapeutic approaches. Additional research is necessary to gain a complete understanding of the intricate interplay of various factors linked to HIF-1. This review provides a relatively detailed account of the regulatory signaling pathway of HIF-1 and its link to the pathogenesis of RA and highlighted several drugs and methods targeting HIF-1 related signaling pathways. Other potential targets, such as PHD-2 and Notch pathways, warrant further exploration, and various HIF inhibitors are continuously evolving. Therefore, future research on RA should focus on controlling the signaling pathway of HIF-1α to inhibit its expression, thereby mitigating a series of pathophysiological changes caused by HIF-1α.
Literature review
We conducted a search on PubMed over the past decade for relevant literature on the relationship between HIF-1 and RA. Our objective was to understand the structure and regulation of HIF-1 and explore its involvement in the occurrence and progression of RA. In order to delve into the intricate relationship between HIF-1 and a specific molecule in RA, we combined these terms in our search. The following keywords were primarily used: HIF-1, RA, FLS, inflammation, angiogenesis, cartilage destruction, VEGF, MMP, Notch and so on. The language restriction for retrieving articles was set to English, and there were no limitations on the types of articles searched.



