Introduction
Adenocarcinoma of the colon and rectum (COAD) is one of the most commonly diagnosed cancers of the gastrointestinal system. It is thought that by 2030 more than 2.2 million new cases will be diagnosed and 1.1 million deaths will occur because of this type of cancer [1]. Unfortunately, despite adequate surgical therapies and chemotherapies, the 5-year survival rate of patients is still low. It is worth noting that the 5-year survival rate for patients diagnosed early is approximately 90%, whereas for patients with late diagnosis it is only 10%. This may indicate that metastasis is a critical cause of death among patients suffering from this type of cancer [2]. Therefore, special attention should be paid to search for factors that may function as prognostic markers for COAD patients.
Acylglycerol kinase (AGK) is a known lipid kinase, which produces lysophosphatidic acid (LPA) from monoacylglycerol. It is widely expressed in the heart, brain, kidney, and muscle [3]. Some studies revealed that AGK is a significant cancer-related gene and is upregulated in many human malignancies, e.g. prostate cancer [4], breast cancer [5, 6], oral squamous cell carcinoma [7, 8], hepatocellular carcinoma [9], and renal carcinoma [10]. In prostate cancer tissues the high expression of AGK is correlated with proliferation and migration [11, 12]. AGK directly interacts with the JH2 domain to relieve inhibition of JAK2 and activate JAK2/STAT3 signalling in oesophageal squamous cell carcinoma (ESCC) [13]. AGK activates JAK2/STAT3 signalling, thus promoting transcription of FOXM1 and eventually enhancing the drug resistance of nasopharyngeal cancer cells [14]. However, the expression pattern and clinical significance of AGK in colon adenocarcinoma patients, especially in individuals living in Europe, remain unclear.
Aim
The current study investigated the expression of AGK protein in colon adenocarcinoma samples from proximal and distal parts of the colon to assess its prognostic significance by correlating its immunohistochemical expression with the clinicopathological variables and survival of individuals living in Europe.
Material and methods
Tissue samples
Tissue specimens were received from 110 colon adenocarcinoma patients who underwent surgical resection at the Municipal Hospital in Jaworzno in 2013–2015. The exclusion criteria were as follows: (1) history of previous malignant disease, (2) familial adenomatous polyposis, (3) inflammatory bowel disease, (4) preoperative anti-cancer treatment, and (5) evidence of distant metastasis. The clinicopathological characteristics obtained from the medical records were as follows: age, gender, location of tumour, grade of tumour differentiation, depth of invasion, angioinvasion, regional lymph node involvement, perineural invasion, operation record, treatment record, recurrence, and vital status at the last follow-up date.
The specimens belonged to 56 men and 54 women (mean age: 63; range: 49–71 years). Tumours were located in the proximal part of the colon in 56 cases and in the distal part of the colon in 54. Three levels of differentiation were used to classify the grading as follows: well differentiated (G1), 44 (40 %) cases; moderately differentiated (G2), 40 (36.36%) cases; and poorly differentiated (G3), 26 (23.64%) cases (Table I).
Table I
Demographic, clinical, and tumour-related characteristics of patients included in the study (n = 110)
| Parameter | N | % | |
|---|---|---|---|
| Age [years]: | |||
| ≤ 62 | 53 | 48.18 | |
| ≥ 63 | 57 | 51.82 | |
| M ± SD | 60.73 ±13.56 | ||
| Me [Q1–Q3] | 63 [49–71] | ||
| Min.–Max. | 34–87 | ||
| Gender: | |||
| Females | 54 | 49.09 | |
| Males | 56 | 50.91 | |
| Location of tumour: | |||
| Proximal colon | 56 | 50.91 | |
| Distal colon | 54 | 49.09 | |
| Rectum | 0 | 0.00 | |
| Grade: | |||
| G1 | 44 | 40.00 | |
| G2 | 40 | 36.36 | |
| G3 | 26 | 23.64 | |
| Depth of invasion: | |||
| T1/T2 | 61 | 55.45 | |
| T3/T4 | 49 | 44.55 | |
| Size of primary tumour [cm]: | |||
| ≤ 9 | 58 | 52.73 | |
| ≥ 10 | 52 | 47.27 | |
| M ± SD | 9.23 ±3.60 | ||
| Me [Q1–Q3] | 9 [6–12] | ||
| Min.–Max. | 3–17 | ||
| Angioinvasion: | |||
| No | 58 | 52.73 | |
| Yes | 52 | 47.27 | |
| Regional LN involvement: | |||
| No | 59 | 53.64 | |
| Yes | 51 | 46.36 | |
| Perineural invasion: | |||
| No | 61 | 55.45 | |
| Yes | 49 | 44.55 | |
Immunohistochemical staining
For the immunohistochemical studies the paraffin-embedded specimens were cut into 4-µm-thick sections, fixed on Polysine slides, deparaffinized in xylene, and rehydrated through a graded series of alcohol. To retrieve the antigenicity, the tissue sections were treated twice with microwaves in a 10 mM citrate buffer (pH 6.0) for 8 min each. Subsequently, sections were incubated with rabbit polyclonal antibody to AGK (final dilution 1 : 500) (Invitrogen; cat. number PA5-28566).
For visualisation of protein expression, the sections were treated with a BrightVision detection system and Permanent AP Red Kit (Zytomed). Mayer’s haematoxylin was used to counterstain the nuclei.
Semi-quantitative analysis of AGK expression in colon adenocarcinoma
The scores were assigned separately for the stained area and for the intensity of the immunohistochemical reaction. Quantification connected to the stained area of the tissue section was performed as follows: (1) < 33% of cells showed immunoreaction, (2) 33–66% of the cells had positive reaction to vimentin, and (3) > 66% of the cells were positive. The intensity of the immunohistochemical reaction was quantified as follows: (1) absent or weak, (2) moderate, and (3) strong. Each tissue section was typified by a final grade derived from the multiplication of the stained area and the intensity of the staining. The AGK expression was considered to be absent/low for grade 1; moderate for grades 2, 3, and 4; and strong for grades 6 and 9.
Survival analysis
Survival analysis was conducted in 110 patients. The survival curves were generated using the Kaplan-Meier method. The overall survival (OS) was defined as the length of time between surgery and death. The follow-up period was 60 months. Patients alive were censored at 5 years.
Statistical analysis
Statistical analyses were conducted using Statistica 9.1 (StatSoft, Poland). The clinical characteristics of the patients in relation to AGK immunoreactivity were assessed by performing the Kruskal-Wallis test and U Mann-Whitney test. Additionally, using Spearman’s rank correlation coefficient, the relationship between the immunoexpression of vimentin and age and grade of tumour differentiation was assessed. The Kaplan-Meier method was used to study survival curves, and the long-rank test to compute differences between the curves.
Results
To investigate the clinicopathological and prognostic roles of AGK expression, the immunohistochemical analysis was performed in colon cancer tumour samples and adjacent non-pathological mucosa (Figure 1). It should be noted that in non-pathological colon mucosa the expression was described as low, whereas low, moderate, and strong levels of AGK expression were demonstrated in well, moderately, and poorly differentiated tumours (Figure 1). Among the 110 samples, 47 (42.73%) showed a low immunohistochemical reaction, 36 (32.73%) demonstrated moderate immunoreactivity, and 27 (24.54%) revealed strong expression of AGK protein. The relationships between AGK levels and each clinicopathological parameter are summarised in Table II. As revealed, the level of the AGK immunohistochemical reactivity was correlated with the grade of the histological differentiation (H [2.110] = 90.451; p < 0.001). A strong expression of AGK protein was detected more frequently in patients with G3 tumours than in patients with G2 tumours. As revealed by the statistical analyses, a significant difference was detected also between the patients with G1 tumour and those with G2. In the G2 patients a strong reactivity was described more frequently. Moreover, the significance was also detected between patients with G1 and G3 tumours. The strong level of AGK expression was also detected in patients with T3/T4 depth of invasion (Z = –3.930, p < 0.001). Furthermore, the patients with the presence of angioinvasion, perineural invasion and regional lymph node involvement demonstrated higher levels of AGK immunoreactivity (all p < 0.001). Interestingly, a correlation was detected between the level of AGK reactivity and the size of primary tumour. Patients with the larger size of tumour (≥ 10 cm) demonstrated higher levels of AGK reactivity. The statistical evaluation of AGK immunoexpression according to age, sex, and tumour location revealed no significant difference among these variables (all p > 0.05; Table II).
Figure 1
Immunohistochemical expression of AGK protein in tumour tissue; A, B – weak expression of AGK protein in colon adenocarcinoma (A) and adjacent non-pathological colon mucosae (B), C, D – moderate expression of AGK protein in colon adenocarcinoma (C) and adjacent non-pathological colon mucosae (D), E, F – strong expression of AGK protein in colon adenocarcinoma (E) and adjacent non-pathological colon mucosae (F)
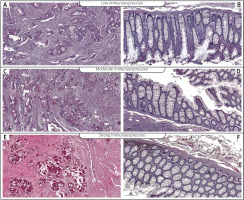
Table II
Correlations between AGK immunoexpression and clinicopathological characteristics in colon adenocarcinoma patients
The Kaplan-Meier survival analysis showed that the 5-year OS rate in the group of patients with low AGK immunoreactivity was significantly longer than that for patients with moderate or strong AGK protein expression (p < 0.001; Figure 2). The mean OS rate was 39.091 months (95 CI: 35.166–43.016), and the median OS rate was 44 months (95 CI: 29.439–58.561). The 5-year OS for patients with a low, moderate, or strong AGK immunoexpression was 78.7%, 13.9%, and 0%, respectively. The low-AGK expression patients had a mean survival time of 59.149 months (95% CI: 58.590–59.708), whereas the strong-AGK expression groups had an average survival time of 13.8015 months (95% CI: 10.367–17.262). In the univariate analysis the age, grade of tumour differentiation, depth of invasion, size of primary tumour, angioinvasion, perineural invasion, regional lymph node involvement, and AGK immunoexpression were found to be significantly associated with reduced 5-year OS. However, a multivariate analysis demonstrated that grade of tumour differentiation (HR = 5.985; 95% CI: 2.901–12.35, p < 0.001), depth of invasion (HR = 0.441; 95% CI: 0.251–0.773, p = 0.004), and AGK immunoexpression (HR = 2.098; 95% CI: 1.168–3.77, p = 0.013) were independent risk factors for worse survival (Table III).
Figure 2
Kaplan-Meier survival curves of colon adenocarcinoma patients with different expression of AGK protein; follow-up period 60 months

Table III
Univariate and multivariate analyses of various prognostic parameters in colon cancer patients using Cox regression analyses
Discussion
As revealed by a great number of studies, the high expression of AGK is correlated with poor clinical outcomes in cancer patients. Therefore, the expression of AGK has emerged as a prognostic factor for patient survival [4–10]. Our immunohistochemical assessment revealed that AGK was strongly expressed in colon adenocarcinoma tissues in comparison to non-pathological colon specimens. Furthermore, the high level of AGK immunoexpression was demonstrated to be clearly corelated with the malignancy-related clinicopathological factors and 5-year OS rate of patients. The high immunoexpression of AGK was significantly related to advanced histological tumour grade. Spearman rank correlation showed a statistical difference between the patients with G1 tumours and those with G2 tumours, and between patients with G1 tumours and those with G3 tumours. Moreover, a difference was noted between G2 and G3 tumours. In these cases, patients with G3 tumours were described by a strong level of AGK as well. It should be noted that a strong level of AGK immunoreactivity was also described in the patients with larger tumour size, advanced pTNM stage, the presence of angioinvasion and perineural invasion, and positive regional lymph node status (all p < 0.001). Importantly, the low-AGK expression group revealed a better prognosis in comparison to the patients with strong expression of this protein. The multivariate analysis revealed that the immunohistochemical level of AGK expression may function as an independent prognostic factor in patients with colon adenocarcinoma.
Several studies revealed that enhanced expression of AGK in cancerous tissue is associated with cell proliferation and tumourigenicity probably via FOXO1 or JAK/STAT signalling [13, 14]. Moreover, Cui et al. reported that AGK activated the NF-κB signalling pathway to enhance angiogenesis and inhibited apoptosis in hepatocellular carcinoma [15]. Murph et al. have demonstrated that LPA, the product of AGK decreased nuclear localization and the presence of the p53 tumour suppressor in A549 lung carcinoma cells [16, 17]. This product may also have an effect on proliferation and invasion in endometrial carcinoma and hepatocellular carcinoma [18, 19]. Ectopic expression of AGK activated gastric cancer cell proliferation and epithelial-mesenchymal transition under in vitro conditions. Interestingly, knockdown of AGK expression showed the opposite results in nude mouse tumour cell xenograft growth. Moreover, in gastric cancer patients AGK expression was associated with tumour cell de-differentiation and poor overall survival [20]. Therefore, AGK may be a potential cancer therapeutic target. The results of our study demonstrated that expression of AGK is clearly upregulated in colon adenocarcinoma samples. Moreover, this expression is related to factors associated with tumour aggressiveness and metastatic abilities.
It should be noted that there were some limitations to our study. First, it should be pointed out there are unidentified and limitless agents such as environmental factors, which might result in a bias. Secondly, our patients were only from the Caucasian population, especially individuals living in Poland. It would be ideal to expand this study among other populations in the future to confirm our findings.










