Introduction
Over the last dozen or so years, the statistics regarding bariatric surgeries have changed. At the beginning of this millennium, Roux-en-Y gastric bypass (RYGB) was dominant, and 20 years later we have the advantage of laparoscopic sleeve gastrectomy (SG) [1]. The SG procedure appears to produce excellent long-term results and low risk of complications [2–4]. The increase in SG performance is influenced by the remission of obesity-related diseases and the stability of weight loss. However, the occurrence of gastroesophageal reflux (GERD) after SG remains controversial and questionable [2, 5, 6].
Typical symptoms of GERD are heartburn, oesophageal chest pain, and regurgitation [7, 8]. According to the Lyon consensus 2.0, endoscopy and/or 24-hour pH-impedance monitoring should be performed to confirm the diagnosis [7]. The Los Angeles (LA) scale is used in gastroscopy [7]. All cases of grade B LA can be diagnosed as GERD [9]. LA-A may occur in up to 7.5% of the population, and in some patients gastroscopy may not show any changes (non-erosive GERD – NERD) [10]. Therefore, further tests are needed to make a diagnosis. PH-monitoring is an objective method to investigate the actual degree of gastric regurgitation. Normal limits include acid exposure time less than 4% (AET < 4%) and less than 40 reflux episodes [11]. The diagnosis is confirmed by pH monitoring above 6% AET and above 80 reflux in a 24-hour test [7]. Due to reports of the occurrence of reflux in patients after SG, we decided to design an objective study assessing the occurrence of reflux de novo. The study included asymptomatic patients with 24-hour pH-monitoring before and 6 months after surgery.
Aim
The aim of the study was to determine the occurrence of GERD after SG. The secondary outcome was early weight loss after the surgery.
Material and methods
Study design
This is a prospective study of patients undergoing SG at a high-volume centre in a European country in 2022. Inclusion criteria included meeting the eligibility criteria for bariatric surgery, no symptoms of GERD, normal gastroscopy, normal pH-monitoring, and no evidence of hiatal hernia during laparoscopy. Exclusion criteria were as follows: unwillingness to participate in the study and follow-up, symptoms of GERD, presence of LA oesophagitis grade A–D, Barrett’s oesophagus, symptoms of hiatal hernia or cardia failure on gastroscopy, more than 6% AET and more than 80 episodes of reflux during pH-monitoring, or symptoms of hernia hiatus intraoperatively.
The group qualified for analysis included 50 patients. The database included patient demographic characteristics (sex, age, maximum weight, preoperative weight, body mass index (BMI)). It also included information on preoperative gastroscopy, preoperative and postoperative pH monitoring, surgery, and the results of bariatric treatment (current body weight and BMI, percentage of total body weight loss (%TWL)). Preoperative examinations were performed up to 1 month before surgery. Postoperative examinations were performed 6 months after surgery. Four patients refused postoperative examination. The follow-up rate was 92%. The study is in line with STROBE guidelines.
Surgical technique
SG was performed according to standard technique [12]. The sleeve was created using a 36Fr calibration cart, starting approximately 3–4 cm proximal to the pylorus and ending approximately 1 cm distal to the gastroesophageal junction to maintain the angle of His. A Signia stapler (Medtronic) was used, starting with the black cartilage, then 2 purple cartilages and 2-3 gold cartilages. Clips were used in the bleeding spots of the staple lines. There was no other staple line reinforcement. No repair of the diaphragm crura was performed during any surgery.
pH-monitoring
24-hour pH-monitoring was performed using single-use catheters with electrode and impedance (Digitrapper pH Z, Given Imaging). The study was performed on an outpatient basis. PPIs were discontinued for at least 14 days before the study. Patients were told not to modify their daily habits. After calibration in buffers of pH 4.0 and 7.0, the catheter was introduced transnasally and positioned with the pH sensor 5 cm above the gastroesophageal junction.
Statistical analysis
A descriptive statistical analysis was conducted. All data were analysed using Statistica software 13.PL (StatSoft Inc.). The minimum required sample size of 37 was calculated using Cochran Sample size formula based on a margin of error of α = 0.05, a confidence level of 95%, and population proportion of 0.1. Continuous values were presented as the mean with standard deviation. Qualitative and quantitative variables were compared using the χ2 or Kruskal-Wallis test when appropriate. Univariate logistic regression was performed to calculate the OR with 95% confidence interval (CI). The Pearson’s correlation between pH-monitoring values and patients characteristics was performed. P-values at less than 0.05 were considered statistically significant.
Ethical considerations
The data were completely anonymised. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. The study was approved by Bioethics Committee of the Military Chamber of Physicians in Poland (20/2022). The study was registered under the number NCT05486169.
Results
Patients’ characteristic
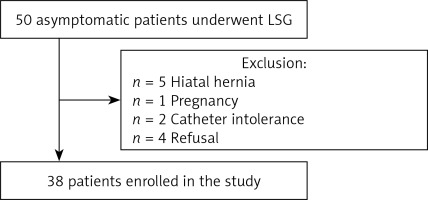
In total, 50 patients met the preoperative inclusion criteria. Twelve patients were excluded from the postoperative pH-monitoring (Figure 1). Five patients were excluded from the study due to the intraoperative diagnosis of hiatal hernia requiring cruroplasty. Two patients were excluded due to catheter intolerance after the surgery. One patient became pregnant in the early postoperative period. Four patients refused postoperative examination, claiming that it was not necessary due to the lack of GERD symptoms. Thirty-eight patients were analysed in the study. There were 28 (73.9%) females. The mean age was 44.9 ±11.0 years. The mean preoperative BMI was 42.6 ±5.0 kg/m2. The mean length of hospital stay was 1.0 ±0.2 days, and the mean operative time was 37.6 ±8.8 min. There were no complications or mortality within the first 6 months after surgery.
pH-monitoring outcomes
Before surgery, all patients had normal pH-monitoring values. After surgery, the mean AET, the number of refluxes, and the DeMeester score increased statistically significantly (p < 0.001) (Table I). Postoperatively, mean values reflected the diagnosis of GERD. Only 11 (28.9%) patients had AET < 6%. Twenty-seven (71.1%) patients had AET > 6%, which met the GERD criteria, but only 9 (23.7%) reported symptoms of GERD and the need for PPIs. There were no statistically significant differences between the diagnosis of GERD and patients’ characteristics (Table II).
Table I
The values of parameters of pH-monitoring before and after the surgery
Table II
Characteristics of patients. Patients are divided into those whose acid exposure time (AET) after the procedure was above 6% and after the procedure below 6%
Moreover, all available factors contributing to < 6% AET after surgery were analysed in univariate logistic regression model. No statistically significant results were found (Table III).
Table III
Univariate logistic regression analysis for factors contributing to AET <6% after the surgery. (AET acid exposure time, BMI body mass index, %EWL percentage of excess weight loss, %TWL percentage of total weight loss)
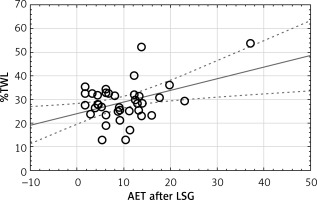
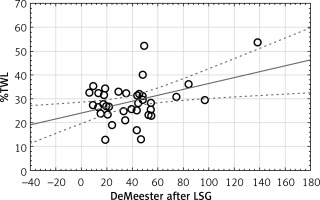
A Pearson’s correlation was performed between pH-monitoring values and patients’ characteristics (Table IV). The correlation between AET and %TWL was moderate positive, and the correlation between DeMeester score and %TWL was low positive (p = 0.011, p = 0.014, respectively) (Figures 2 and 3).
Table IV
The Pearson correlation between pH-monitoring values (AET, RFX, DM) and patients’ characteristic. (AET acid exposure time, RFX refluxes, DM DeMeester score, preBMI preoperative body mass index, %EWL percentage of excess weight loss, %TWL percentage of total weight loss). Statically significant, bold
| Variable | Age | P-value | preBMI | P-value | %EWL | P-value | %TWL | P-value |
|---|---|---|---|---|---|---|---|---|
| AET | –0.061 | 0.714 | 0.109 | 0.515 | 0.242 | 0.143 | 0.409 | 0.011 |
| RFX | –0.059 | 0.723 | 0.139 | 0.405 | 0.146 | 0.380 | 0.287 | 0.080 |
| DM | –0.054 | 0.746 | 0.133 | 0.423 | 0.220 | 0.184 | 0.397 | 0.014 |
Discussion
This prospective observational study aimed to assess the real occurrence of de novo GERD in patients after SG. The study, which involved pre- and post-operative pH-monitoring, revealed that over two-thirds of patients met the criteria for GERD after the surgery.
There is no clear cause of GERD after SG; it is a multifactorial disease. Lower oesophageal sphincter (LES) is one of the most important components of the anti-reflux barrier. The occurrence of reflux after SG is associated with an increase in transient LES relaxations (TLESR) lasting several seconds [13]. Some authors attributed this phenomenon to the shortening of the LES resulting from the surgical technique used and a neuromediated reflex initiated by postprandial gastric distension [14]. Others look for problems in oesophageal motility disorders. Another factor predisposing to the development of GERD after surgery is the disruption of another point of the anti-reflux barrier, which is the angle of His. The closer to the angle of His we move the stapler, the more it will disturb its functions, which will result in a greater risk of GERD after surgery [15]. Moreover, the shape of the remaining stomach may be responsible for the occurrence of GERD [13]. Any narrowing in the sleeve may cause increases in pressure above it, and thus symptoms of reflux.
In the 10-year follow-up of the SLEEVPASS randomised clinical trial, the incidence of oesophagitis after SG was 31% [2]. Moreover, as many as 64% of patients must continue to take PPIs after surgery, and in almost 50% symptoms worsened after surgery. Yeung et al. prepared a meta-analysis on reflux after GERD [16]. According to their study, 23% of patients experience de novo reflux after surgery, and up to 30% experience oesophagitis. These data are heterogeneous but do not change the reality that GERD after SG is a problem.
Sethi et al. demonstrated on a small number of patients who they examined before surgery using pH-monitoring [17] that those with a positive preoperative test had a higher risk of conversion to bypass after SG compared with patients who were also symptomatic before the procedure but had negative pH-monitoring. Soliman et al. came to similar conclusions [18]. A larger study than the previous one showed that the correlation of preoperative symptoms with a positive pH-metric test is also a risk factor for the occurrence of GERD symptoms after surgery.
Few authors published the results of an analysis of preoperative and postoperative objective tests in patients regardless of symptoms of GERD [19, 20]. Poggi et al. performed pH measurements in 60 patients before and 12–24 months after surgery [19]. Before surgery, 35% of patients had an elevated DeMeester score, and after surgery it increased to 83.3% (from 16.71 ±12.78 to 42.88 ±32.08). De novo reflux was found in 79.48% of patients. Gemici et al. showed a statistically significant difference in AET before and 3 months after SG in 62 patients (5.06 ±4.14 vs. 9.84 ±8.09) [20]. The data provided are consistent with ours.
Completely opposite results were presented by Castagneto-Gissey et al. [21]. They observed no significant statistical differences between preoperative and postoperative examinations in patients after SG. A trend towards improvement can even be observed (AET < 4% 10.7 vs. 4.4; number of refluxes 78.8 vs. 40.7). However, they observed a significant increase in oesophagitis before and after the surgery (10.5% vs. 42.1%). According to their study, pH-monitoring is not a good test for assessing reflux after SG due to differences in its results and those in imaging tests. Better postoperative pH-metric results were also observed by Chern et al.; however, 20 out of 25 patients analysed by them had synchronous hiatal hernia repair and fixation performed [22]. Nonetheless, this is contrary to the results of other studies [23].
A very interesting work was published by Thereaux et al. [24]. They examined patients with normal and abnormal pH-monitoring results before and after surgery. In the group of 21 patients with normal pH-metry, AET increased significantly after SG (1.6 vs. 5.6). However, 19 patients initially diagnosed with GERD achieved statistically insignificant improvement (7.7 vs. 5.9). Patients with visible intraoperative hiatal hernia had it repaired at the same time. This is consistent with the results of our study that in patients initially asymptomatic and with normal pH-metry, the parameters significantly deteriorate.
This study has strengths and stands out among other works. All patients analysed in this study had no subjective or objective symptoms of GERD before surgery. We also excluded patients with anatomical abnormalities, such as intraoperative observation of a hiatal hernia. The surgeries were performed by a team of 2 surgeons working together, performing over 500 operations a year, which minimises the risk of intraoperative differences and analysis bias. The person performing pH-monitoring also had a few years of experience in conducting over 100 tests per year.
Our paper also has some limitations. We analysed a small group of patients, but the group was homogeneous, which can be applied to a broader population of patients. Moreover, we did not assess GERD preoperatively using a questionnaire or other tests including manometry, nor did we perform endoscopic examinations after surgery. In the future, it is worth presenting the results of studies in these patients, including imaging tests and longer follow-up.