Summary
Currently, easily accessible and effective scoring systems are still needed to estimate the severity and prognosis of coronary artery disease. Therefore, in this study we aimed to evaluate the usefulness of the advanced lung cancer inflammation index, which can be obtained with a simple calculation, through its relationship with the SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) score.
Introduction
The SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) score (SXscore) is an angiographic scoring tool used to determine the extent and severity of coronary artery disease (CAD) [1]. The prognostic value of the SXscore has been demonstrated in patients with acute coronary syndrome (ACS) [2–4], and the SXscore is also used to determine the revascularization strategy.
Inflammatory markers have been shown to play an important role in the pathogenesis and progression of atherosclerosis [5]. The advanced lung cancer inflammation index (ALI) is an independent prognostic biomarker of inflammation and nutrition in various types of cancer, acute heart failure and ACS [6–13]. It is calculated by the formula body mass index (BMI) × serum albumin concentration/neutrophil-to-lymphocyte ratio (NLR). The NLR is a systemic marker of inflammation associated with SXscore, mortality and cardiac events in patients with ACS [14–16]. Serum albumin, as a negative acute phase reactant and nutritional biomarker, is found at lower levels in the case of the increased inflammatory response and is inversely related to SXscore and mortality in patients with ACS [17, 18]. Also, BMI has been reported to have an inverse relationship with SXscore [19].
Aim
In this study, we aimed to examine the relationship between ALI and a high SXscore in patients admitted to our hospital with non-ST elevation myocardial infarction (NSTEMI).
Material and methods
Retrospectively, patients who were admitted to our hospital due to NSTEMI and underwent coronary angiography between February and November 2020 were consecutively included in the study. The patients’ demographic, laboratory, and angiographic data were obtained using the hospital database. Patients with coronary artery bypass grafting (CABG), severe kidney and liver dysfunction, active infection, chronic inflammatory disease, and malignancy were not included in the study. Of the 313 patients screened, 17 were excluded due to CABG, 6 had severe renal dysfunction, 1 had severe liver dysfunction, 2 had a history of malignancy, 3 were excluded due to a lack of laboratory data, and 284 patients were included in the study.
Cases of patients with ischemic symptoms and without ST-segment elevation on electrocardiography but with classical increases or decreases in cardiac troponin I were defined as NSTEMI. Hypertension (HT) was defined as blood pressure higher than 140/90 mm Hg on repeated measurements or antihypertensive drug use with a history of HT. Diabetes mellitus (DM) was defined as fasting blood glucose ≥ 126 mg/dl, or ≥ 200 mg/dl in any measurement, or antidiabetic drug use with a history of DM. Hyperlipidemia was defined as a fasting total cholesterol (TC) level > 200 mg/dl or a previous diagnosis and treatment for hyperlipidemia. A family history of CAD was considered present if there was a history of sudden cardiac death or CAD in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula [20]. ALI was calculated with the following formula: BMI × serum albumin/NLR, which were calculated from patients’ admission values. Other routine laboratory parameters were recorded. BMI, serum albumin, NLR and other laboratory data (except peak troponin I) were obtained from blood sampling before coronary angiography.
All patients underwent coronary angiography using the standard Judkins technique. The SXscore I was calculated by an independent cardiologist who was unaware of the clinical characteristics of the patients, based on the online SXscore calculator according to the patients’ coronary anatomical features (www.syntaxscore.org). SXscore I was calculated for all coronary lesions ≥ 50% diameter stenosis in vessels ≥ 1.5 mm. Patients with a myocardial infarction with non-obstructive coronary arteries (MINOCA) were not included in the study. An occluded culprit artery was scored as an occluded artery with < 3 months duration. SXscore I was divided into two groups, low (< 32) and high (≥ 33), and analyzed. Multivessel coronary artery disease (MVD) was defined as luminal stenosis of at least 70% in at least two major coronary arteries or in one coronary artery in addition to a 50% or greater stenosis of the left main trunk. All patients were administered aspirin and a P2Y12 inhibitor (clopidogrel or ticagrelor) as antiplatelet therapy, and unfractionated or low molecular weight heparin as anticoagulant therapy. The decisions of revascularization strategies or medical follow-up were left to the discretion of physicians.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (IBM Corp.). Continuous variables were presented as mean ± standard deviation; categorical variables were presented as percentages. The Kolmogorov-Smirnov test was used to verify the normality of the distribution of continuous variables. The independent sample t-test or the Mann-Whitney U test was used for continuous variables and the χ2 test for categorical variables. Variables with a significant correlation with a high SXscore in univariable regression analysis were included in the multivariable regression analysis to determine the independent predictors of a high SXscore. P < 0.05 was considered the statistical significance level, and the confidence interval (CI) was set at 95%. Receiver operating characteristic (ROC) analysis was used to determine the cutoff levels of ALI to predict a high SXscore.
All procedures were carried out in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki.
Results
The mean age of the patients was 63.9 ±12.2 years. The mean age was higher in the group with a high SXscore (70.1 ±8.9 vs. 63.6 ±12.3, p = 0.042). Table I shows patients’ demographic, clinical, and biochemical parameters with low and high SXscores. BMI was lower in patients with a high SXscore (25.6 ±4.7 vs. 28.6 ±4.9, p = 0.02). The two groups were similar in terms of male gender, DM, HT, current smoking, hyperlipidemia, family history of CAD, and previous myocardial infarction. NLR was higher in the group with a high SXscore (4.7 ±1.2 vs. 3.0 ±2.5, p = 0.01). Serum albumin was lower in the group with a high SXscore (3.9 ±0.3 vs. 4.3 ±0.4, p < 0.001). Patients with a high SXscore had lower ALI (22.4 ±7.3 vs. 58.5 ±44.3, p = 0.016). C-reactive protein (CRP) was higher in the group with a high SXscore (6.6 ±4.9 vs. 3.2 ±3.7, p = 0.001), and peak troponin was higher in the group with a high SXscore (26.1 ±44.5 vs. 10.4 ±16.6, p = 0.002). Other biochemical parameters such as serum glucose, serum creatinine (CR), eGFR, LDL-C, high-density lipoprotein cholesterol (HDL-C), TC, TG, white blood cell (WBC) count, and hemoglobin were similar in the two groups. MVD was higher in the group with a high SXscore (15 [100%] vs. 109 [40.5%], p < 0.001). Treatment methods differed between groups. CABG was higher in the group with a high SXscore (8 [2.9%] vs. 8 [53.3%], p < 0.001).
Table I
Demographical, clinical, and biochemical values
[i] BMI – body mass index, CAD – coronary artery disease, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, ALI – advanced lung cancer inflammation index, NLR – neutrophil-to-lymphocyte ratio, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting, LMCA – left main coronary artery.
According to the median value of ALI, the population was divided into low and high ALI groups. Table II shows baseline characteristics of the low and high ALI groups. The mean age (66 ±12 vs. 62 ±12), BMI (26.7 ±4.4 vs. 30.1 ±4.6), WBC count (9.6 ±2.9 vs. 8.7 ±2.5), NLR (4.5 ±2.9 vs. 1.8 ±0.5), serum albumin level (4.1 ±0.4 vs. 4.4 ±0.4), serum CR level (1.0 ±0.6 vs. 0.9 ±0.2), TG level (148.7 ±99.9 vs. 184 ±142.5), CRP level (4.3 ±4.2 vs. 2.4 ±3.1), and peak troponin I level (14.5 ±23.2 vs. 7.9 ±13.8) were significantly different between the low and high ALI groups (all p < 0.05). Low ALI was significantly associated with high SXscore (13 [9.1%] vs. 2 [1.4%], p < 0.001). MVD (90 [63.3%] vs. 34 [23.9%], p < 0.001) and left main coronary artery (LMCA) disease (7 [4.9%] vs. 2 [1.4%], p < 0.001) were higher in the low ALI group. CABG was higher in the low ALI group (14 [9.8%] vs. 2 [1.4%], p < 0.001).
Table II
Baseline characteristics of low and high ALI groups
[i] BMI – body mass index, CAD – coronary artery disease, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, ALI – advanced lung cancer inflammation index, NLR – neutrophil-to-lymphocyte ratio, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting, LMCA – left main coronary artery.
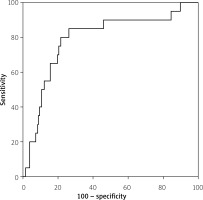
In the univariable analysis, age (p = 0.046), BMI (p = 0.021), C-reactive protein (p = 0.002), peak troponin I (p = 0.009), NLR (p = 0.025), albumin (p = 0.003) and ALI (p < 0.001) were significantly associated with a high SXscore (Table III). Since ALI itself includes NLR, albumin and BMI, two models were created for multivariable analysis. Model A includes NLR, albumin, BMI, peak troponin I, CRP and age. Model B includes ALI, peak troponin I, CRP and age. Model B revealed ALI (95% CI: 0.931–0.984, p = 0.002) as an independent determinant of a high SXscore (Table III). In ROC curve analysis for prediction of a high SXscore, an optimal cutoff value of 31 for ALI had 80% sensitivity and 78% specificity (area under curve = 0.791, 95% CI: 0.681–0.900, p < 0.001; Figure 1).
Table III
Univariable and multivariable analysis of predictors of High SYNTAX Score
| Variables | Univariable analysis | Multivariable analysis Model Aa | Multivariable analysis Model Bb | |||
|---|---|---|---|---|---|---|
| Odds ratios (95% CI) | P-value | Odds ratios (95% CI) | P-value | Odds ratios (95% CI) | P-value | |
| Age | 1.046 (1.001–1.099) | 0.046 | 1.016 (0.972–1.062) | 0.48 | 1.027 (0.986–1.070) | 0.2 |
| Gender | 1.075 (0.331–3.487) | 0.9 | ||||
| Diabetes mellitus | 0.773 (0.256–2.337) | 0.65 | ||||
| Hypertension | 0.771 (0.272–2.186) | 0.62 | ||||
| Family history of CAD | 1.754 (0.482–6.282) | 0.4 | ||||
| Previous MI | 0.889 (0.284–2.778) | 0.77 | ||||
| Current smoking | 0.447 (0.149–1.343) | 0.15 | ||||
| BMI | 0.856 (0.750–0.977) | 0.021 | 1.203 (0.881–1.193) | 0.75 | ||
| Admission serum glucose | 1.001 (0.994–1.008) | 0.75 | ||||
| Serum creatinine | 1.025 (0.345–3.049) | 0.97 | ||||
| Hyperlipidemia | 0.949 (0.293–3.077) | 0.93 | ||||
| White blood cell count | 1.123 (0.960–1.313) | 0.14 | ||||
| LDL-C | 1.006 (0.994–1.019) | 0.31 | ||||
| Triglyceride | 0.999 (0.994–1.004) | 0.67 | ||||
| Hemoglobin | 0.952 (0.690–1.313) | 0.77 | ||||
| Peak troponin I | 1.023 (1.006–1.040) | 0.009 | 1.015 (0.994–1.035) | 0.16 | 1.010 (0.993–1.028) | 0.25 |
| C-reactive protein | 1.170 (1.059–1.292) | 0.002 | 1.011 (0.883–1157) | 0.88 | 0.994 (0.882–1.119) | 0.91 |
| Serum albumin | 0.684 (0.451–0.797) | 0.003 | 1.012 (0.986–1.037) | 0.07 | ||
| NLR | 1.158 (1.018–1317) | 0.025 | 1.023 (0.998–1.049) | 0.07 | ||
| ALI | 0.919 (0.880–0.961) | < 0.001 | 0.957 (0.931–0.984) | 0.002 | ||
Discussion
This study examined the relationship between ALI and high SXscore in patients with NSTEMI. In the present study, ALI was significantly associated with the extent and complexity of coronary artery disease, as measured by the SXscore, in patients with NSTEMI.
Although many modern therapeutic advances have been made today and the incidence of adverse cardiovascular outcomes has decreased, ACS remains the leading cause of mortality and morbidity worldwide [2, 21]. For this reason, there are many risk scores for risk classification in determining the prognosis and managing patients [22]. The SXscore helps to determine the extent and severity of coronary lesions, and its value in risk stratification in the prognosis and management of patients with ACS has been repeatedly demonstrated [23–25]. Factors affecting the severity of coronary lesions are inflammatory markers [26, 27] and atherosclerosis risk factors (such as HT, advanced age, DM, and cigarette smoking) [28, 29].
ALI is an easily accessible and inexpensive marker calculated by NLR, BMI, and serum albumin levels. These are the parameters associated with the extent and severity of coronary artery disease, represented by the SXscore. ALI was first proposed in 2013 as an independent prognostic marker of inflammation in patients with metastatic non-small cell lung cancer [6] because systemic inflammation is associated with cancer development and poor outcomes. Later, its prognostic importance was confirmed in various types of cancer and acute heart failure [7–12]. So far, numerous data support the key role of inflammatory mechanisms in the development of atherosclerosis [30–32]. ALI is a biomarker of inflammation and nutrition because it includes NLR, BMI, and serum albumin levels. A recent study reported the prognostic importance of ALI in ACS patients undergoing PCI [13]. This study showed that ALI, a novel inflammation index, can effectively predict the risk of major adverse cardiovascular events in patients with ACS undergoing PCI. As mentioned above, the role of inflammatory mechanisms in the development of atherosclerosis and the prognostic value of SXscore in ACS patients have been demonstrated. However, no studies have investigated the association of ALI with the extent and severity of atherosclerosis.
There is increasing clinical evidence highlighting the prognostic importance of inflammation in the development of coronary artery diseases in both acute and chronic conditions [33, 34]. It has been shown that inflammatory parameters such as tumor necrosis factor α, interleukin (IL)-1, IL-6, and CRP are elevated in atherosclerotic heart disease [31, 35], and reducing the inflammatory response has slowed the development of atherosclerosis and decreased cardiovascular events [36]. It has recently been reported that WBC count and leukocyte subtype cells are useful in reflecting the inflammatory process in atherosclerotic heart diseases [37]. Studies have shown that WBC count is an independent predictor of death in acute myocardial infarction [38]. Neutrophils are a subtype of WBC in the blood and have an important role in the inflammatory response [39]. These cells may contribute to the development of atherosclerosis by secreting certain cytokines and enzymes [40, 41]. Lymphocytes have regulatory immune response properties and can slow down this process through some secretory compounds [41]. Low lymphocyte count has been associated with the progression of atherosclerosis [38].
NLR is calculated by dividing the neutrophil count by the lymphocyte count. This ratio is an easily accessible inflammatory marker associated with the extent and severity of coronary artery disease and has predictive power for high-risk CAD [37, 42]. Albumin is considered a negative acute phase protein. Inflammation has been associated with decreasing synthesis and increasing catabolism of albumin [43]. It has been reported that decreased admission serum albumin concentration is associated with higher SXscores and in-hospital mortality in patients with ACS [17, 18]. Although some studies have reported obesity as an independent risk factor for coronary artery disease [44–47], recent studies suggest that, unlike previous studies, obesity is not a risk factor for CAD and may have protective effects on the progression of CAD, known as the “obesity paradox” [29, 30]. A more recent study showed a negative correlation between BMI and the severity of CAD [19]. In our study, ALI as a combination of NLR, serum albumin level, and BMI was found to be inversely related to a high SXscore in patients with NSTEMI. Additionally, this study found that ALI alone was an independent predictor of a high SXscore in multivariable analysis.
This study has some limitations. First, this study is retrospective. Second, the number of patients is relatively small. Third, patients with CABG were not included in the study because the SXscore could not be calculated. Fourth, only patients with NSTEMI undergoing coronary angiography were included in this study. Therefore, this study is not representative of all ACS patients.