Introduction
The increasing number of device implantations including pacemakers, implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy (CRT) devices necessitates implementation of the best lead management strategy to avoid procedure-related complications. The complications can be divided into infectious (lead-related infective endocarditis, pocket infection or both) or noninfectious (lead dysfunction, mechanical lead damage at various levels, cardiac perforation, lead-induced tricuspid valve dysfunction, etc.). Broadly speaking, the management strategy also involves decisions whether to extract or abandon functional but superfluous leads, or to implant new ones if a device upgrade is needed. The more time the pacemaker is in the body the more decisions we have to make regarding its various parts. Replacement of a pacing unit may be relatively easy, but it is much more challenging to extract or replace the leads that have been implanted for a number of years or even for decades and may be ingrown by scar tissue in the veins and surrounding cardiac structures.
The importance of close collaboration between the cardiologist and the cardiac surgeon during transvenous lead extraction has been emphasized in the professional society guidelines for some time now [1, 2]. This collaboration is especially valuable when life-threatening complications arise and only emergency cardiac surgery can save the patient’s life. For this reason the cardiac surgeon should be available during the extraction procedure either directly in the operating room or on call, together with the whole team and adequate equipment.
However, the role of a cardiac surgeon is not limited to rescue interventions in case of life-threatening complications.
Aim
The aim of this study is to identify various areas of collaboration between the cardiac electrophysiologist and the cardiac surgeon in lead extraction or, broadly speaking, in lead management.
Material and methods
Data collected during 3207 lead extraction procedures performed by the team of the same experienced operator in two centers between 2005 and 2019 were reviewed. The team performs 230 extractions a year on average, meaning that it is one of the most experienced lead management centers – according to the guidelines, high-volume centers are those performing > 30 transvenous lead extraction (TLE) procedures annually [3]. Extractions for noninfectious indications were done in 66.4% of patients, whereas 33.6% of patients had their leads removed because of infection. The mean dwell time of extracted leads was 91.5 months (> 7.5 years). According to the current guidelines [2] the proper definitions of success were used – complete procedural success, which means the removal of all targeted leads and material with the absence of any disabling complications or procedure-related death, was achieved in 96.1% of patients and clinical success, which means retention of a small portion of a lead (less than 4 cm long) without a negative impact on the outcome (risk of perforation, embolic events, perpetuation of infection) with the absence of any disabling complications or procedure-related death was achieved in 97.8%. The need of rescue surgical intervention does not exclude procedural success, if it does not end with a disability or procedure-related death. Procedure-related mortality was 0.18%. Major complications occurred in 1.9% of procedures and included acute tamponade (1.2%), hemothorax (0.2%), tricuspid valve damage (0.3%), stroke and pulmonary embolism (< 0.2%).
Results
Cardiac surgical intervention was necessary in 80 (2.49%) patients. In some of them during surgical intervention with sternotomy due to a life-threatening complication or the necessity of complementing partially successful transvenous lead extraction the implantation of an epicardial pacing system was required. For the purpose of this analysis implantation of this epicardial system was treated as another surgical procedure in the same patient. Also some of these patients underwent a second late surgical intervention mainly due to the increase of TLE-related tricuspid valve dysfunction. Ultimately a total of 113 various cardiac surgical procedures were performed in the study population. Based on the analysis of these procedures five areas of collaboration between the cardiac electrophysiologist and the cardiac surgeon during transvenous lead extraction were identified.
Immediate surgical intervention in patients with major life-threatening complications
In the study group surgical rescue interventions were required during 38 (1.18%) from among 3207 procedures. Details for all surgical interventions are provided in Table I. Right atrial wall damage during atrial lead extraction was the main reason for immediate cardiac surgical intervention (24/38, 63.1%). Other sites of perforation in this study were the superior vena cava (occasionally with bleeding into the right pleural cavity), coronary sinus (when extracting a left ventricular lead) or right ventricular wall. Occasionally, bleeding may occur from two sites at a time. Other, much less frequent causes of immediate cardiac surgical intervention were massive pulmonary embolism (1 patient), and extensive damage to the tricuspid valve requiring immediate repair (1 patient). In 1 case due to circulatory collapse of unknown etiology and ineffective resuscitation it was decided to perform emergency exploratory sternotomy. Direct cardiac massage combined with visual control of filling and contractility provided stabilization, and ten days later the patient was discharged home in a good general condition.
Table I
The role of cardiac surgery in TLE procedures
[i] A – atrial, V – ventricle, RA – right atrium, RV – right ventricle, VCS – vena cava superior, CS – coronary sinus, TV – tricuspid valve, TVD – tricuspid valve dysfunction, TVP – tricuspid valve plasty, TVR – tricuspid valve replacement, EPI – epicardial, LV – left ventricle, VVI – single chamber ventricular pacemaker, DDD – dual chamber pacemaker, CRT-P – cardiac resynchronization therapy with a pacemaker, CRT-D – cardiac resynchronization therapy with defibrillator, BiV – biventricular, HV – high-voltage defibrillator lead, ENDO – endocardial.
Late cardiac surgical intervention in TLE-related tricuspid regurgitation
Among 3207 procedures 14 (0.44%) patients required late surgical intervention because of TLE-related tricuspid valve dysfunction (TLE-related TVD) – 11 patients required tricuspid valve repair, 2 patients received tricuspid bioprosthesis, and in 1 patient tricuspid valve replacement was performed twice in 14 years.
Cardiac surgical intervention complementing partially successful TLE
In this study 22 (0.68%) patients required cardiac surgery complementing partially successful lead extraction (Table I).
Epicardial pacemaker placement during surgery with sternotomy
In the present study 21 (0.65%) patients required an epicardial pacemaker during emergency or late sternotomy for the reasons discussed above. Type of pacing and number of epicardial leads are presented in Table I.
Discussion
The cardiac surgeon has a crucial role in the management of life-threatening complications of TLE, which is highlighted in the professional society guidelines and confirmed in many studies [1–4]. Damage to the thoracic great veins or cardiac walls is the most dangerous complication arising from the extraction procedure that may cause acute cardiac tamponade and rapid circulatory decompensation. In such cases only emergency sternotomy, immediate evacuation of pericardial blood and stopping the bleeding may save the patient’s life. It is assumed that from the onset of circulatory decompensation the cardiac surgical team has only about 5–10 minutes to correct the problem [4]. If this time interval is exceeded, there is a dramatic increase in the risk of severe neurological injury and death.
Cardiac surgical intervention for a life-threatening condition must be immediate and efficient, which is why adequate organization of TLE procedures is the key to success. It should provide:
early recognition of complications, even before the signs of hemodynamic instability are identified (the key role of continuous transesophageal echocardiographic monitoring (TEE), which can detect tamponade within 30–60 seconds before hypotension occurs in the course of the procedure);
immediate start of cardiac surgical intervention; for this reason general anesthesia is recommended in each patient undergoing TLE with the presence of an anesthesiologist and nurse experienced in cardiac surgical interventions, with direct arterial blood pressure monitoring and venous access (in the case of cardiorespiratory instability we gain valuable time that otherwise would be used for intubation and anesthesia for rescue sternotomy);
immediate availability of the qualified cardiac surgical team consisting of:
the cardiac surgeon performing lead extraction arm in arm with the cardiologist, wearing a sterile surgical gown and standing at the table until the procedure is completed, ready to start sternotomy immediately after recognition of acute tamponade,
the second cardiac surgeon on call who can join the team at the table within 3–5 minutes after being summoned (until that time the cardiologist assists the cardiac surgeon with sternotomy and bleeding management),
the scrub nurse (present on site who is able to scrub and put on a sterile gown within 1 minute),
the assisting nurse, not wearing a sterile gown, who helps connect necessary equipment (suction pump, electrocautery machine, sternal saw) and provides adequate instruments and surgical materials,
the perfusionist making available an extracorporeal circulation machine within minutes;
immediate availability of adequate equipment:
surgical tools with a sternal saw, covered with a drape and laid out on a sterile table (in the corner of the operating room) ready for immediate use during each extraction procedure,
cautery machine assuring quick and effective control of surgical bleeding,
suction pump available for quick and effective evacuation of blood from pericardial sac or pleura,
cardiopulmonary bypass for use if needed,
cell saver device to collect blood lost during surgery, set up in advance to ensure that it is available quickly for emergency evacuation of acute tamponade and bleeding management; drainage of the pericardium should not be delayed until the cell saver is ready, as hemodynamic recovery is the more urgent priority;
availability of cross-matched blood units prepared and on standby (routine procedure in each patient qualifying for TLE).
In this study right atrial wall damage during atrial lead extraction was the main reason for rescue surgical intervention (63.1%). There are two sites and mechanisms of this damage. The first is perforation of the right atrial appendage at the site of positioning the tip of the atrial lead. This site is easy to identify and surgically manage. As the site of perforation is within the atrial appendage, a mobile part of the right atrial free wall, it can be quickly located after sternotomy and pericardial incision and easily clamped to prevent further loss of blood. To this aim one can use curved vascular clamps or partial aortic clamps commonly used for aortic cross clamping during coronary bypass grafting. Such clamps should always be available in the surgical toolkit during TLE procedures. After clamping the appendage the perforation can be easily repaired using non-absorbable sutures. The second site of possible right atrial damage is the right atrium-right ventricle interface – right atrioventricular groove (right coronary sulcus). This injury is much more difficult to manage. As a result of exaggerated rotation of the appendage during lead extraction maneuvers the atrium may partially be torn off from the ventricular wall. Bleeding from such a tear may be more massive than after perforation of the appendage. The limited possibilities of clamp placement at this site of injury and the risk of damage to the neighboring structures (including the right coronary artery running through the right atrioventricular groove) make it much more difficult to control the situation. Temporary manual compression at the site of bleeding by the cardiac surgeon may help, allowing time for choosing the most effective treatment option and preparation of the most appropriate tools and sutures. It is also time for replenishment of volemia, and setting up and priming the cell saver for blood collection.
The key is to restore the blood circulation as soon as possible. All logistic preparations mentioned above concerning availability of proper equipment and medical staff should help to do this. During each TLE procedure the cardiac surgeon involved in the TLE team must be ready for immediate sternotomy. Usually in the case of acute cardiac tamponade to attempt the pericardiocentesis is a waste of time – even 5–10 minutes of delay can cause irreversible neurological dysfunction or death because of cerebral ischemia. If a cardiac surgeon is not present during the TLE procedure, in the case of circulatory decompensation it is reasonable to try a pericardiocentesis for at least temporary improvement until cardiosurgical intervention is possible. Only immediate sternotomy and opening the pericardial sac make it possible to find the cause of tamponade and to promptly stop the bleeding. In most cases there was no need to use cardiopulmonary bypass (CPB) – firstly because the atrial or ventricle wall damage can be quickly identified and sewn up and secondly, in such rescue cases usually there is no time to establish it. More extensive vein wall damage, especially caused by laser-assisted extraction devices (not used in this study group), may require the CPB support for its reconstruction [5, 6].
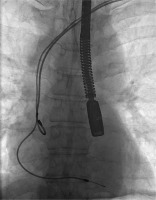
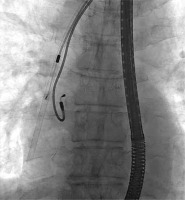
Figures 1–6 present the successive stages of the TLE procedure with atrial perforation effectively treated by the cardiosurgical team.
Figure 3
The atrial lead was damaged during attempts of its preparation from surrounding scar tissue by rotational maneuvers of non-powered mechanical sheath
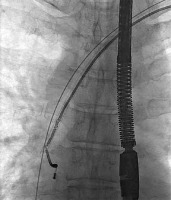
Figure 4
The atrial lead was successfully removed in spite of its damage and stretching – a short while later TEE showed increasing fluid in pericardial sac
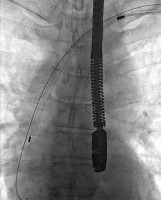
Figure 5
Immediate cardiosurgical intervention with sternotomy because of acute tamponade due to right atrial appendage perforation – the perforation was rapidly sewn up without CPB use
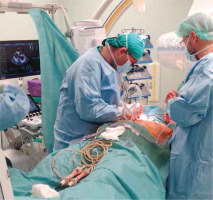
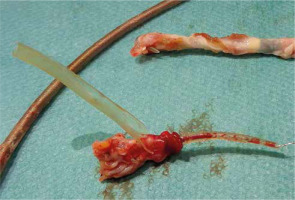
Figure 6
The tip of the ventricular lead with scar tissue (above) and atrial lead (below) with fragment of right atrial appendage wall and destroyed lead covering
Late cardiac surgical intervention in lead-induced tricuspid regurgitation
A lead implanted transvenously into the right ventricle passes through the tricuspid valve and becomes a potential source of valvular dysfunction through compression on valve leaflets (mainly septal), entanglement with the chordae tendineae or leaflet perforation. Even an optimal placement of the lead in the commissure between the septal and anterior leaflet may limit tricuspid annulus mobility during heart work, causing with time annular dilatation and tricuspid regurgitation. A defibrillation lead, thicker and stiffer, may impair tricuspid function to a greater extent. Similarly, a larger number of leads introduced through the tricuspid orifice (for instance when placing an additional right ventricular lead if the non-functional one is abandoned) and loops of mainly atrial leads bulging into the right ventricle may negatively affect tricuspid function over time. These mechanisms lead to the development of various degrees of tricuspid regurgitation in patients with pacemakers or ICD devices [7]. Al-Mohaissen and Chan [8] observed an increase in tricuspid regurgitation after pacemaker implantation by at least one grade in 24.2% of patients, and by at least 2 grades in 18.3% of patients. Initially, lead-induced tricuspid regurgitation is asymptomatic, but with time it may cause severe right ventricular failure, which is associated with a worse prognosis among patients with cardiac implantable electronic device (CIEDs).
Prolonged interaction between the leads crossing the right atrioventricular orifice and tricuspid valve results in tissue fibrosis, stiffening, calcification and degeneration, which impairs leaflet mobility and causes progressive valvular dysfunction. This is followed by the development of fibrotic attachments between chronically implanted leads and the valvular structures, leaflet fusion, shortening of the chordae tendineae, fibrosis and shortening of the papillary muscles. Not only various degrees of tricuspid regurgitation but also tricuspid stenosis may occur. These mechanisms may contribute to deterioration of the tricuspid valve function after transvenous lead extraction. The need to dissect the lead from structural elements of the tricuspid valve using closed techniques, under control of only fluoroscopy and possibly transesophageal echocardiography, may promote further worsening (in 5.6–9.1% of patients undergoing transvenous lead extraction [9, 10]), sometimes leading to acute postprocedural regurgitation requiring cardiac surgical repair. In this study involving 3207 TLE procedures severe tricuspid valve regurgitation due to its rupture during the procedure occurred in 0.50% (16/3207) of cases, whereas postprocedural worsening of tricuspid regurgitation defined as an increase in regurgitation by 2 grades was found in 2.1% of patients. It is clearly less as compared to other studies. One reason may be the optimal organizational model of lead extraction procedures in our center. Each patient undergoes echocardiography just before TLE to evaluate the tricuspid function, and immediately after the procedure to estimate the actual impact of the extraction on the tricuspid valve. General anesthesia providing comfort both to the patient and the operator facilitates continuous transesophageal echocardiographic monitoring of the procedure. Any sign of tricuspid dysfunction during the procedure, valve rotation, pulling on or fusion of the leaflets can be noted by the echocardiographist, who advises the operator to correct the maneuvers to limit the destructive impact of lead extraction on the structural components of the tricuspid valve. Perhaps the type of tools used for extraction also matters; however, there are not sufficient data to compare the effect of specific techniques on postprocedural tricuspid damage.
Cardiac surgical intervention complementing partially successful TLE
Occasionally, removal of an entire lead is not feasible and lead fragments are left behind. This may contribute to cardiac dysfunction (for instance rhythm disturbances or tricuspid valve impairment) or cause other problems, mainly infectious complications. In such cases surgical intervention is performed immediately during TLE or at a later time electively or after the development of new clinical symptoms. Large vegetations in lead-related infective endocarditis may also require surgical removal and at that time surgical treatment may be in the form of:
a planned hybrid procedure, i.e. TLE followed by planned surgical removal of vegetations and/or replacement/repair of the infected heart valves on the left or right side using extracorporeal circulation;
unplanned surgical intervention after the TLE procedure during which it was impossible to remove all vegetations or large residual vegetations and/or fragments of the infected leads left in the heart; surgical intervention may be performed during the same hybrid operation or delayed until new clinical symptoms develop (for instance sepsis recurrence).
Epicardial pacemaker placement during surgery with sternotomy
The placement of the epicardial leads may be necessary during emergency or complementary surgery with sternotomy. A decision about the necessity of epicardial pacing is made by the cardiologist, but this is the cardiac surgeon who will be implanting leads during surgery. Screw-in or suture-on, mono- and bipolar epicardial leads are available. Epicardial leads are required to be present in the operating room – the number and type of epicardial leads should be checked before each TLE procedure. When suturing the lead on the epicardium the surgeon must cooperate with the cardiologist, who decides on the mode of pacing, number and type of leads, and optimal lead location on the myocardium and then verifies the parameters of pacing. In rare cases, the cardiac surgeon must change the location of the lead several times to obtain optimal pacing parameters.
Placement of left ventricular epicardial leads
After TLE some patients may require the placement of a left ventricular epicardial lead – either for permanent cardiac pacing in the VVI mode or as a component of the CRT device. In 5–10% of cases it is not possible to implant a left ventricular lead through the coronary sinus [11], and alternative approaches should be considered. Additionally, after extraction of the old LV lead, reimplantation via the same coronary sinus may be difficult (adhesions in the CS ostium, fibrosis or mechanical damage to the veins in the LV). Available evidence shows a similar effectiveness of epicardial LV pacing in CRT devices as compared to traditional endocardial pacing [12].
Role of the cardiac surgeon in prevention and treatment of local and generalized infections after TLE
The risk of local infection at first pacemaker implantation is estimated to range from 1% to 4% [13] and increases with subsequent interventions in the pacemaker pocket. Evidence shows a high rate of bacterial contamination on implantable devices – even up to 50% [14]. Ultimately, relatively few patients appear to have developed overt clinical signs of local or generalized infection, but the above data are puzzling. A similar rate of local postoperative wound infection (1–4%) is found in patients after surgical operations [15], which take much longer than pacemaker implantation and involve a larger surgical field. Based on surgical experience we may reduce the number and extent of pocket infections, mainly during subsequent lead extractions. To this aim, one should consider the following suggestions:
Change the venue for lead extraction from the electrophysiology laboratory (without the strict operating room hygiene routine) to a hybrid room or operating room where the principles of asepsis are more rigorous than in the cardiological operating rooms (clean room, airlock, change of gown, laminar air flow, appropriate preparation of the patient for entry to the surgical theatre, etc.).
Use the surgical knowledge and experience in aseptic practices during extraction procedures (proper hand washing, correct wearing of surgical caps and masks, principles of behavior at the surgical table, etc.), in protection of the surgical field (for instance surgical foil, drapes covering most of the patient’s body), surgical techniques for pocket incision, dissection of the device and the leads (use of surgical cautery), adequate control of bleeding, and in management of the infected wound.
Be familiar with types of technical equipment that can be used during the procedures (surgical sutures, hemostatic materials, surgical instruments).
If there is suspicion of early superficial wound infection in the pocket, consult appropriate management with a surgeon (swabs, wound decontamination and preparation, antibiotic treatment, etc.).
All these factors may affect the rate and severity of infectious complications after TLE and the rate of other local complications (hematomas in the pocket, scar aesthetics, surgical wound dehiscence). The use of surgical techniques and equipment may reduce periprocedural tissue injury and shorten the duration of the procedure. Consequently, adequate collaboration between the cardiologist and the cardiac surgeon may affect late outcomes after TLE.
Conclusions
In our judgment, the above discussed areas of collaboration between the cardiologist and the cardiac surgeon have a significant effect on the outcomes of transvenous lead extraction, especially in the case of severe complications requiring immediate cardiac surgical intervention. Similarly, surgical knowledge and experience may help reduce the risk of infection and local hematomas after TLE, shorten the duration of the procedure and reduce perioperative tissue injury. This study also shows that active participation of cardiac surgeons in the lead management team may help solve other problems if endocardial pacing is not possible.