Introduction
Non-small cell lung cancer (NSCLC) is a term that encompasses several types of lung cancer. The main types of NSCLC include adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [1]. In developed countries, the mortality rate for LC is around 3-4 times higher than that of developing nations [2].
Transforming growth factor β1 (TGF-β1) is an immune protein that regulates many immune cell types’ generation and effector functions. It can control the innate and adaptive immune systems [3]. TGF-β1 may promote the growth and differentiation of tumour cells through autocrine or paracrine activity, ultimately resulting in increased cell-matrix interaction, inhibition of immune surveillance, or increased angiogenic activity [4]. However, in the early stages of cancer, TGF-β1 acts as a tumour suppressor by inhibiting cellular proliferation or promoting cellular apoptosis [5-7].
The TGF-β family ligands primarily regulate gene expression by receptor-mediated activating mothers against decapentaplegic homolog 2 (SMAD) proteins, which is context-dependent. Various ways of control feed into the SMAD proteins to modulate the signal amplitude, duration, and specificity as essential mediators of TGF-β signalling. SMAD proteins are essential for cell proliferation, differentiation, migration, and apoptosis. Distinct levels of SMAD family members have been observed in various tumours and may be used as prognostic biomarkers in some malignancies [8].
The deregulation of the Wingless-related integration site Wnt/β-catenin signalling pathway is closely related to the initiation and progression of various cancers [9, 10]. Wnt/β-catenin is an evolutionarily conserved cellular signalling network that regulates endothelial cell differentiation and vascular remodelling during embryonic and adult development; a gain of function mutation of β-catenin in endothelial leads to vascular remodelling [11]. Canonical Wnt/β-catenin signalling involves developmental and physiological processes, including apoptosis, cell proliferation, survival, polarity, differentiation and migration invasion, and tissue homeostasis. Its dysregulation leads to various developmental and physiological processes of diseases, including cancer [12-14]. The canonical Wnt pathway is activated by the Wnt protein Wnt3a [15].
β-catenin is a multifunctional and evolutionarily conserved molecule known as armadillo in Drosophila and plays a critical role in a wide range of developmental and homeostatic processes in metazoans [16]. β-catenin also controls cell-to-cell adhesion by binding to cadherin cell adhesion molecules and providing a link to the actin cytoskeleton [17].
In this study, we conducted comprehensive testing of biomarkers (TGF-β1, SMAD2, Wnt3a, CTNN-β1) to assess their diagnostic, prognostic, and screening capabilities for NSCLC.
Material and methods
Patient sampling and setting of the study
This cross-sectional study was conducted at the Hiwa Cancer Teaching Hospital in Sulaymaniyah City, Iraq, from September 2020 to November 2021. Ninety-three patients with newly diagnosed NSCLC (stages III and IV) who were not receiving chemotherapy or radiotherapy were enrolled to participate in this study after oral consent [18]. As controls, 84 healthy non-smoking subjects with no clinical evidence of cancer also participated in the study. Information on smoking history and family history of lung cancer was collected from all participants (patients and HCs). In the patient group staging was done using the Tumor Nodes Metastasis (TNM) classification (8th ed.) of lung malignancies [19], and the symptom score was assessed according to the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines [20]. The study was approved by the Research Ethics Committee of the College of Medicine, University of Sulaimani (REC-230-1/9/2020), and the Medical Ethics Committee of Hiwa Cancer Teaching Hospital. Therefore, the research related to human use has complied with all the relevant national regulations and institutional policies that follow the tenets of the Helsinki Declaration. Informed consent was obtained from all the patients enrolled in the current study.
Outcome measures
Five millilitres of blood were collected from the peripheral veins of patients and HCs, then transferred into a gel tube, left at room temperature for 20 minutes to coagulate, and then centrifuged at 4500 RPM for 10 minutes to separate the serum sample. The sera samples were collected and stored in a freezer at –70°C until the day of sample analysis. To analyse serum biomarker (Wnt3a/CTNN-β1 TGF-β1/SMAD2) production, we conducted an enzyme-linked immunosorbent assay (ELISA) using the sandwich method on the serum sample using a commercially available kit (catalogue numbers: E-EL-H5681, E-EL-H5623, E-EL-0162, and E-EL-H5623) respectively (Elabscience Biotechnology Inc., Texas, USA) based on the manufacturer’s protocol [21]. C-reactive protein titre (CRP) was analysed with a Cobas c111 analyse (Roche Diagnostics), lactate dehydrogenase (LDH) with Cobas c111 analyses (Roche Diagnostics), and erythrocyte sedimentation rate (ESR) with the Westergren method [22].
Statistical analysis
For data analysis, statistical analysis was performed using GraphPad Prism 9.4 Software (GraphPad, La Jolla, California, USA). The clinical characteristics and demographic attributes of individuals were evaluated using the chi-square (χ2) test. Statistical differences in the serological data between patients and HCs were determined using the Mann-Whitney U test. D’Agostino and Shapiro-Wilk normality tests were performed for all data. The area under the curve (AUC) was used to analyse the association between biomarkers’ concentration and NSCLC using the ROC curve [23]. The markers’ median and interquartile range (IQR) were calculated. Correlations were analysed between TGF-β1, SMAD2, Wnt3a, and CTNN-β1 in the serum of NSCLC patients and were calculated using the Spearman correlation test [24]. The χ2 test was employed to determine the relationship between categorical variables. A p-value < 0.05 was considered statistically significant.
Results
Population characteristics
There was no significant difference in age and sex between the patient group and the HCs (Table 1). Around half of the patients with NSCLC were diagnosed with squamous cell carcinoma in TNM stage III, and most were active smokers (Table 1).
Table 1
Demographic characteristics of non-small cell lung cancer (NSCLC) and healthy controls (HCs)
Biomarker comparison between groups
Regarding biomarkers, the NSCLC group had significantly higher levels of all the measured biomarkers than the HC group (Table 2). In the NSCLC group, there were no significant differences in biomarkers between those with adenocarcinoma and squamous cell carcinoma (Table 3).
Table 2
Comparison between the median of participants with detectable biomarkers between non-small cell lung cancer (NSCLC) and healthy controls (HCs)
Table 3
Comparison between adenocarcinoma (AD) and squamous cell carcinoma (SQ) in patients with non-small cell lung cancer (NSCLC)
The level of biomarkers was non-significant in NSCLC patients between stage IV and stage III (Table 4). In gender, there were no notable differences in the levels of remodelling and inflammatory biomarkers in the NSCLC, except for Wnt3a (Table 5).
Table 4
Comparison of biomarkers between stage III and stage IV in patients with non-small cell lung cancer (NSCLC)
Table 5
Comparison between males and females in patients with non-small cell lung cancer (NSCLC)
Biomarker correlations
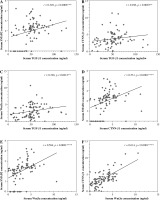
A positive correlation between TGFβ1 and SMAD2, TGFβ1 and CTNN-β1 and TGFβ1 and Wnt3a in the NSCLC group was found in (Fig. 1A-C). There was also a statistically significant positive correlation between CTNN-β1 and SMAD2, Wnt3a and SMAD2, and CTNN-β1 and Wnt3a in patients with NSCLC (Fig. 1D-F). However, there was no significant correlation between symptom scores and biomarkers (Table 6).
Fig. 1
Correlation between serum-biomarkers: A) between SMAD2 and TGFβ1, B) between CTNN-β1 and TGFβ1, C) between Wnt3a and TGF-β1, D) between CTNN-β1 and SMAD2, E), between Wtn3a and SMAD2, F), between CTNN-β1and Wnt3a in non-small cell lung cancer
ROC biomarker analysis between MSCLC and HCs
The ROC curve and AUC profiling methods were employed to analyse the sensitivity and specificity of the remodelling and inflammatory biomarkers in relation to NSCLC. The CTNNβ1 and Wnt3a serum levels were determined to have higher accuracy in predicting NSCLC, as illustrated in Figure 2A-D.
Discussion
The major result of this study is that patients with NSCLC had higher levels of inflammatory and remodelling biomarkers than HCs. Furthermore, in the NSCLC, there were significant associations between the inflammatory and remodelling biomarkers.
In the present study, the levels of TGF-β1 were significantly higher in the NSCLC group than in the HC group. This is in accordance with previous studies that have reported that the level of TGF-β1 protein in NSCLC patients was significantly higher than in normal lung tissues [25]. TGF-β1 has many biological activities that participate in regulation and homeostasis within cells and tissues as signalling factors in apoptosis. In addition, TGF-β1 also has a role in the overgrowth of cells and serves as an enhancer during cancer development [26].
In our study, the NSCLC group had higher levels of SMAD2 than the HC group. In a previous study, no significant difference was observed in the production of SMAD2 between lung cancer and normal tissues in the same person [27]. The disparity in these results could be due to different methods for diagnosing this biomarker and assessing its level in two individuals. SMAD2 is a SMAD family eukaryotic transcription factor activated via phosphorylation after the TGF-β family generates cell surface receptors. Unlike other SMAD families, SMAD2 does not bind with DNA during activation. Instead, SMAD2 is responsible for transducing the signal into the nucleus, leading to gene activation in haematopoiesis, differentiation, and apoptosis [28, 29].
In our study, the levels of Wnt3a were significantly higher in the NSCLC patients than in the HC group. Other studies have shown that Wnt3a level increases in colon carcinomas [15]. Wnt3a is a secreted type of glycoprotein that binds to cell surface receptors and activates a cascade of intracellular events that lead to the formation of several proteins that play an important role in tissue homeostasis, embryonic development and regulating bone mass [30, 31]. We also found higher levels of CTNN-β1 in the NSCLC group than in the HC group. The present results agree with the studies of patients with colorectal cancer [32]. The role of Wnt3a and CTNN-β1 in non-small cell lung cancer has not been widely studied in humans. β-catenin is a component of the adherens junction-forming cadherin-associated protein complex. The adherens junctions’ cadherin-associated protein complex contains β-catenin. It is also critical to the Wnt/β-catenin signalling pathway. β-catenin affects cell differentiation, proliferation, apoptosis, metastasis, and tumorigenesis [33].
The NSCLC group had a significant correlation between the TGF-β1 with SMAD2, Wnt3a, and CTNN-β1. Interaction between inflammatory and remodelling biomarkers’ physiological mechanisms supports the hypothesis that these biomarkers’ Wnt3a induced TGF-β1 level and signalling through SMAD2 in a CTNN-β1 mechanism [34]. This holds great promise for future pharmacologically targeted NSCLC therapy.
The study has several limitations. The small sample size is one factor that can lead to a higher standard error rate. Due to the exclusion of individuals who had undergone previous treatments, the sample size was limited. Blood samples were taken only at one point; regularly re-assessing the biomarkers could have led to higher precision. The smoking practices of individuals with lung cancer were not uniform. While tobacco usage is recognized to impact an individual’s immune system, it is worth noting that other variables, such as the environment, should also be taken into account. The research solely examined individuals who had undergone a resection procedure.
Conclusions
This study found that patients with NSCLC had higher inflammatory and remodelling biomarkers than HC. Furthermore, in the NSCLC, there were significant associations between the inflammatory and remodelling biomarkers. This indicates that measuring biomarkers could be valuable in the workup of NSCLC patients. Our investigation showed that inflammatory and remodelling biomarkers might play a role in future immunologic response and pharmacologically targeted NSCLC therapy.



