Introduction
Inflammatory bowel disease (IBD) is a chronic disease characterized by dysregulation of the immune system with exaggerated response to surrounding stimuli. Crohn’s disease (CD) and ulcerative colitis (UC) are the most common forms of IBD [1].
Those patients are exposed to frequent procedures and hospital admission as well as recurrent need for radiological examinations with a high risk of exposure to radiation and contrast. Guidelines for diagnosis and staging of degree of disease activity have evolved over time [2].
Blood tests such as C-reactive protein (CRP) and faecal calprotectin can indicate the presence of inflammation, but they do not provide information about the location or severity of inflammation [3, 4]. Endoscopy, on the other hand, can visualize the lumen of the digestive tract and identify areas of inflammation, but it is an invasive procedure that requires sedation and can be uncomfortable for the patient [5]. Laboratory tests and endoscopy can provide useful information about the degree and the extent of inflammation in the digestive tract, but they have several constraints when it comes to evaluating the response to biologic therapy [6].
Imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) can be used to evaluate the response to biologic therapy [7, 8]. They can detect complications of the disease such as abdominal abscesses, fistulae, or strictures [9]. However, these techniques are expensive, expose the patient to ionizing radiation, and may not provide sufficient detail to assess changes in inflammation over time. The need for non-invasive and objective method for follow-up is still required after proper validation and correlation to other modalities of diagnosis.
Recently, growing evidence in bowel ultrasonography has become more evident by the increase in the number of communications and clinical trials over the last decades [10, 11].
Because of its affordable price, absence of side effects, and easy accessibility, intestinal ultrasound (IUS) has established itself as a promising bedside technique for tracing clinical activity in patients with inflammatory bowel disease [12]. It is becoming more widely accepted as a weapon in the diagnostic toolbox for IBD, as well as for determining the severity of the inflammation, early identification of complications, either related to the disease or to the therapeutic protocol; with results equivalent to other validated techniques.
We still need solid results to encourage transition of the use of such a technique from large-volume referral or research centres to a daily practice procedure in IBD clinics [13, 14].
Aim
Our objective was to evaluate the role of a combination of both IUS and colour Doppler with different parameters for monitoring changes in inflammation over time and to reveal their potential ability in the assessment of response to biologic therapy in IBD patients and in predicting which patients are likely to respond to treatment with anti-tumour necrosis factor (anti-TNF).
Material and methods
Patient selection
This cohort research was conducted between June and November 2022 at the IBD Clinic. Based on laboratory, colonoscopy, and histological data, 45 individuals with confirmed IBD were included. We identified individuals who had never been treated with a biologic drug before (bio-naive). Anti-TNF was administered to all eligible patients in accordance with the established guidelines. Each patient’s indication for anti-TNF treatment was validated by the IBD committee.
In addition to baseline laboratory data such as complete blood count, liver and kidney function tests, C-reactive protein, erythrocyte sedimentation rate (ESR), and faecal calprotectin, all patients had a comprehensive history review.
Before the induction of biological therapy, intestinal ultrasound together with colour Doppler of the intestine was conducted. Response to therapy was defined following the guidelines of the European Crohn’s and Colitis Organization (ECCO).
Patients with a history of cancer, liver cirrhosis, renal failure, or heart failure, and patients who had symptoms of infections in more than one organ were excluded from this study.
All included patients provided informed permission and signed assent to participate in the investigation, follow the prescribed treatment, and report any adverse effects linked to the condition or treatment. Prior to the beginning of patient recruitment, the research protocol was authorized by the relevant institutional ethics committee.
Intestinal ultrasound and colour Doppler test
All examinations were performed by single experienced radiologist, to eliminate the inter-observer variation. The operator was blinded to the clinical, laboratory, and endoscopic data of the patients except for any relevant information for the diagnosis. We used different probes for the test: a convex 7-MHz probe and a superficial curvilinear 15-MHz probe, for visualization of organs. We also applied colour Doppler for evaluation of splanchnic vessels. Any detected abnormality was reported, such as abdominal lymph nodes enlargement, ascites, or abscesses. The bowel wall thickness was measured on fixed cross section images of different bowel segments. We calculated the peak systolic velocity (PSV), end diastolic velocity (EDV), and resistivity index (RI) of the superior mesenteric artery. The units were as follows: PSV cm/s, EDV cm/s, and R.I. is a ratio.
Statistical analysis
Version 18 of the Statistical Package for the Social Sciences (SPSS) software was employed. All factors are described in terms of the mathematical mean plus or minus the variance. The observed continuous data representing 2 classes were compared using Student t-tests. χ2 evaluation was conducted to examine discrete elements. Using the receiver operator characteristics (ROC) model, the validity of PSV and RI in forecasting the response to therapy in IBD patients was investigated. A p-value lower than or equal to 0.05 was deemed significant in statistical terms.
Results
A total of 45 patients with histopathological diagnosis of IBD were enrolled in the study. All patients received biological therapy and were assessed for response after 3 months. Out of those patients, 34 (75.6%) had good response while 11 (24.4%) failed to respond to the treatment (Table I).
Table I
Baseline data of the studied patients based on response to therapy
Patients with good response had insignificantly lower mean age (29.35 ±5.30 vs. 31.36 ±7.25 years; p = 0.32). Majority of patients (33 patients) had ulcerative colitis while 12 patients had luminal Crohn’s disease. Based on clinical, laboratory, and histopathological grading of the activity, 73.5% of patients with good response had initial mild and moderate disease activity at the start of the therapy, while the majority of patients (72.7%) who failed anti-TNF treatment after 3 months had initially severe activity, with a significant difference between the groups (p = 0.02) (Table II).
Table II
Baseline laboratory and ultrasound finings based on response to therapy
Patients with good response had significantly higher mean platelet volume (9.48 ±2.06 vs. 7.56 ±1.68 fl; p < 0.001). Meanwhile, the failure group had significantly higher PSV (134.27 ±13.16 vs. 105.82 ±32.80; p < 0.001) and RI (0.76 ±0.05 vs. 0.71 ±0.06; p = 0.02). Other laboratory and radiological data showed no significant differences between both groups (Table III).
Table III
Accuracy of PSV and RI in the prediction of response to therapy
| Parameter | PSV | RI |
|---|---|---|
| Sensitivity | 73% | 64% |
| Specificity | 79% | 82% |
| Positive predictive value | 44.4% | 54% |
| Negative predictive value | 89% | 87.1% |
| Accuracy | 77.5 | 77.6% |
| Cut-off point | > 134 | > 0.76 |
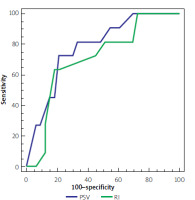
| Area under curve | 0.78 | 0.71 |
| P-value | < 0.001 | 0.01 |
At cut-off > 134, PSV had 77.5% overall accuracy for prediction of the response to therapy with an area under curve of 0.78, while at cut-off > 0.76, RI had 77.6% overall accuracy for prediction of the response to therapy with an area under curve of 0.71 (Figure 1).
Discussion
Inflammatory bowel disease is a chronic disease that affects millions of patients across the world, causing inflammation and damage to the digestive tract, and leading to a major impact on public health systems worldwide [15]. Biologic therapy has become increasingly popular in recent years as an effective weapon in the management of IBD, particularly in those with moderate to severe symptoms or those who failed to respond to prior conventional therapy. The use of biologic therapy has been regulated by setting clear specific targets that have an impact on the clinical course of the disease, leading to improvement in the quality of life of IBD patients [16].
Regular medical monitoring and close assessment of the degree and activity of bowel inflammation are required for effective control of the disease [17]. On the other hand, evaluation of the response to biologic therapy can be challenging. As a result, IBD specialists used to rely on objective measures such as laboratory tests, endoscopic examination, and radiologic evaluation to assess the efficacy of this treatment [18, 19]. These tools have several limitations. The use of endoscopy to monitor the response to therapy in IBD is limited by the difficulty of repeating this invasive examination frequently [16]. Repeated exposure to contrast material together with ionizing radiation has been reported in IBD patients [20, 21]. That is where intestinal ultrasound and colour Doppler play a major role. They offer a reliable, safe, non-invasive method for the determination of evolution of the disease severity comparable to other cross sectional imaging tests [22, 23]. They may provide the appropriate tool for assessment of the usefulness of biologic therapy by measuring specific signs of inflammation in the digestive tract and allow for early detection of complications in this specific population (fistula, stricture, or abscesses) [24, 25]. Several groups investigated the use of intestinal ultrasound to predict the response to biologic therapy in IBD patients [26]; still, these results need to be confirmed on large case-control studies to validate each sonographic parameter.
Intestinal ultrasound uses high-frequency sound waves while using probes with different depths of penetration of abdominal tissues and organs. The received signal image can provide detailed information about the thickness of the intestinal wall, the presence of ulcers or strictures, enlarged mesenteric lymph nodes, as well as the location and extent of inflammation [27]. Colour Doppler is a type of ultrasound that can measure blood flow in real-time. By using colour-coded images, it can detect abnormalities in the pattern of blood flow in mesenteric vessels (mainly the superior mesenteric artery), which can correlate with the presence of active inflammation [28].
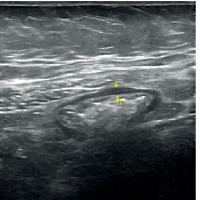
Bowel wall thickness (BWT) is the most employed ultrasonography measure in IBD patients, probably as a result of the impact of the inflammatory activity on the integrity of the intestinal mucosa. According to several reports, a BWT of more than 4 mm might imply the presence of severe ulcerative colitis that is actively developing [29]. The TRUST-UC trial is the broadest multicentre investigation concerning the application of intestinal ultrasound in patients with UC; in this study, BWT was verified as a US parameter for the identification of disease activity [30]. Intestinal ultrasound has been compared to MRI for assessment of activity in IBD patients and showed good agreement [31]. Figure 2 showing intestinal ultrasound photo with increased thickness of the ascending colon in patient with inflammatory bowel disease.
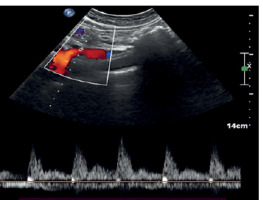
Colour Doppler plays a major role in the evaluation of splanchnic haemodynamics and particularly in inflammatory intestinal disease [32]. There have been observations of enhanced vascularity in the intestinal walls in severe IBD patients [33]. By taking several measurements of the superior mesenteric artery, such as velocity, RI, or flow volume, many research teams have managed to characterize the disease’s activity [27] (Figure 3).
Figure 3
Colour Doppler of the superior mesenteric artery in an IBD patient: PSV = 73, EDV = 25, and RI = 0.66
It has been suggested that BWT and mesenteric vascular Doppler could be utilized simultaneously to improve the diagnostic accuracy in IBD patients [10]. Several validated scoring indices along with endoscopy as a reference standard incorporated intestinal ultrasound with colour Doppler [34–36]. We have tried to show a clear relationship between the combination of IUS with colour Doppler and the response to anti-TNF therapy.
Colour Doppler US can also be adapted for slow flow detection, which is useful to define bowel wall flow (BWF) and to assess vascularity in thickened bowel wall.
The role of colour Doppler associated with IUS has been verified in the evaluation of the response to therapy and has been evaluated essentially through the correlation with anti-TNF through levels [37]. We have studied the intestinal US findings combined with the vascular flow changes in the superior mesenteric artery (PSV, EDV, and RI).
Our findings point to a strong association between BWT and serum albumin, ESR, and CRP. Combining those elements may predict the serological response in IBD patients under biologic therapy.
The presence of disease activity is suggested in most patients by the combination of changed colour Doppler signal (CDS) and gut wall thickening. The benefit of this objective combination is that it offers excellent operator reliability [38]. The overall sensitivity of IUS varied from 54 to 93% when evaluating bowel affection, with a specificity of 97–100% [39], in comparison to our results, with sensitivity 64–74% and specificity of 79–82%. Doppler parameters could predict the response to the biologic therapy in IBD patients. This can be added to the tools that can serve for following this population.
The reported results in our investigation should be interpreted in the context of 2 limitations. First, intestinal ultrasound is a real-time operator-dependent examination, which may restrict the capacity to recalculate measurements and compare values. The second limitation is the retrospective study design, which constrained our analysis to retrieval of the available data from patients’ records. We recommend conducting further prospective case-control large-scale studies to identify the predictive variables for response that could be detected early by intestinal ultrasound.
Integration of intestinal ultrasound and colour Doppler into clinical practice requires specialized training and equipment. However, several organizations have developed guidelines for their use in IBD patients [40]. By measuring changes in blood flow and inflammation in the digestive tract, these techniques can help healthcare providers determine whether a patient is responding to biologic therapy and whether adjustments to the treatment plan are necessary.
Conclusions
Intestinal ultrasound and colour Doppler provide a safe, non-invasive way to monitor changes in inflammation and blood flow in the digestive tract, allowing healthcare providers to make informed decisions about the treatment protocol. As technology continues to advance, the accuracy and accessibility of intestinal ultrasound and colour Doppler are likely to improve, making these techniques even more useful in the management of IBD. For IBD patients, the use of intestinal ultrasound and colour Doppler can provide reassurance that their treatment is effective.
Overall, the integration of intestinal ultrasound and colour Doppler into clinical practice has the potential to improve outcomes and quality of life for IBD patients, and healthcare providers should consider these imaging techniques as part of their treatment plans.