Summary
This retrospective cohort study examined the prognostic significance of pericoronary fat thickness (PFT) in patients undergoing percutaneous coronary intervention (PCI) for chronic total occlusions (CTO). The study included 415 patients who underwent coronary computed tomography angiography and coronary angiogram. PFT measurements were significantly different among the nonobstructive, PCI (non-CTO), and CTO-PCI groups. Patients in the CTO-PCI group had a 13.9% mortality rate over a median follow-up of 16.6 months, with higher average-PFT values associated with increased mortality. Cox regression analysis identified average-PFT as an independent predictor of mortality, alongside left ventricular ejection fraction and albumin levels. These findings highlight PFT as a potential inflammatory marker and prognostic indicator post-PCI for CTO, suggesting its integration into risk assessment models for improved clinical outcomes.
Introduction
Coronary artery disease (CAD) is the leading cause of mortality worldwide [1]. Approximately 15% of CAD patients have at least one coronary artery completely occluded [2, 3]. Successful revascularization of the chronic total occlusions (CTO) through percutaneous coronary intervention (PCI) requires careful procedural planning and highly skilled physicians [4, 5]. Although there have been notable improvements in angina relief and ischemic burden, there are still limited data on long-term prognosis following these interventions [6, 7].
Inflammation is crucial in the development of CAD, evidenced by immune cells in atherosclerotic plaques [8]. Research indicates that around 60% of myocardial infarctions happen in patients with non-significant coronary artery disease, resulting from plaque rupture in heavily inflamed vascular atherosclerotic territories [9, 10].
Significant amounts of interleukin-6, plasminogen activator inhibitor-1, free fatty acids, and tumor necrosis factor-α can be produced by visceral adipose tissue, and these substances accelerate the onset of atherosclerosis, plaque instability, and arterial thrombosis [11, 12]. Furthermore, the adventitia is strongly linked to epicardial adipose tissue (EAT), which surrounds coronary arteries. EAT may aggravate vessel wall inflammation, which in turn promotes the progression of atherosclerosis [13, 14].
Currently, there are no standardized recommendations for measuring EAT [15]. The need for a more specific imaging modality to determine the coronary inflammation has led to the increased use of pericoronary fat thickness (PFT). PFT is the adipose tissue which interacts closely with adjacent coronary arteries [16, 17].
The literature indicates a clear relationship between PFT and the presence and severity of CAD. However, whether PFT is crucial in determining the prognosis of patients after CTO-PCI remains unexplored.
Aim
This study investigated the relationship between PFT and the prognosis of patients who have undergone CTO-PCI.
Material and methods
Study population
A total of 471 patients who underwent coronary angiogram (CAG) between November 2019 and February 2022 and underwent CT coronary angiography before the procedure were retrospectively screened. Exclusion criteria included malignancies and inflammatory diseases (n = 12), hypersensitivity to contrast agents and systemic disorders, such as thyroid disorders (n = 15), and an inappropriate computed tomography (CT) coronary angiogram (n = 29). After exclusion, 415 patients formed the cohort. Patients were categorized into three groups: the normal group, the PCI (non-CTO) group, and the CTO-PCI group. Additionally, within the CTO-PCI group, a detailed evaluation based on survival status was conducted to explore potential prognostic implications. Baseline demographic and laboratory data were retrieved from the hospital’s electronic database system. The Multicenter Chronic Total Occlusion Registry of Japan (J-CTO) score incorporates five predictors of guidewire passage within 30 min: blunt proximal cap, bending > 45°, occlusion > 20 mm in length, presence of calcification in the CTO lesion, and a previous failed PCI attempt [18]. The EuroCTO (CASTLE) score incorporates factors such as history of coronary artery bypass grafting (CABG), age (≥ 70 years), stump anatomy (blunt or invisible), degree of tortuosity (severe or unseen), occlusion length (≥ 20 mm), and severity of calcification [19]. The Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS-CTO) score aggregates four factors associated with technical failure: proximal cap ambiguity, absence of interventional collaterals, vessel tortuosity, and attempting to treat a left circumflex CTO. Based on the operator’s assessment, interventional collaterals are deemed crossable with a guidewire and micro-catheter [20]. This study was approved by the Local Ethics Committee and conducted in accordance with the guidelines set forth in the Declaration of Helsinki.
CT coronary angiogram and assessment of PFT
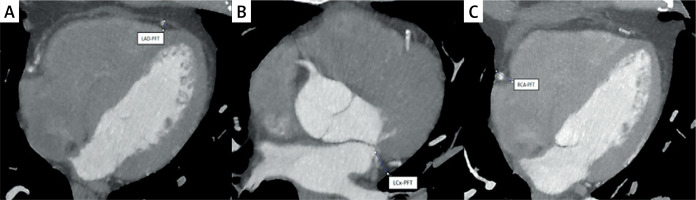
CT coronary angiogram imaging was performed using a 256-slice CT scanner (GE Revolution Evo 256-slice). Imaging was performed during the mid-diastolic phase, chosen for its minimal cardiac motion, with cardiac gating set at 70–80% of the R-R interval. Window settings were meticulously adjusted to enhance visualization of adipose tissue and pericardium. Each scan preceded a contrast-enhanced coronary angiogram using 32 × 0.6 mm collimation, with a tube current of 60 mAs and 120 kV. Patients maintained sinus rhythm during the procedure, and those with heart rates above 60 were given β-blockers to optimize image quality. An intravenous injection of nonionic iso-osmolar contrast medium (Visipaque 320 mg/ml) was administered via the test bolus technique: a 10 ml bolus of contrast agent at 5 ml/s, followed by 50 ml of saline into the antecubital vein, with sequential images captured every 2 s at the aorta and pulmonary arteries. The delay time from injection to peak dye intensity in the aorta was calculated, and after confirming the ECG trigger, image acquisition began with a 60 ml Visipaque 320 mg/ml injection at 6 ml/s, followed by 60 ml of saline at the same rate using a power injector or infusion syringe. Pericoronary fat thickness (PFT) was measured from the myocardium’s outer edge to the visceral pericardium on axial views, explicitly measuring the maximum fat thickness around the left anterior descending coronary artery (LAD), left circumflex artery (LCx), and right coronary artery (RCA) perpendicularly (Figure 1) [21]. The average PFT was determined by calculating the mean thickness of pericoronary fat around these three coronary arteries. Two radiologists with expertise in cardiac imaging, blinded to patient information, independently evaluated all CT coronary angiogram images.
Patient management
The percutaneous coronary intervention (PCI) was carried out using established methods, following a standard protocol for antiplatelet therapy and periprocedural anticoagulation. The decision-making process, including considerations for bilateral injection, retrograde approaches, wire selection, microcatheter usage, and the type of drug-eluting stents, was left to the discretion of the treating physician. Throughout the follow-up period, optimal medical therapy (OMT) for secondary prevention was in line with established guidelines. The treating physician determined the length of dual antiplatelet therapy for patients with drug-eluting stents.
Statistical analysis
SPSS Statistics software, version 21.0 (IBM Corp.), was used for data analysis. The normality of continuous variables was evaluated using the Kolmogorov-Smirnov test. For continuously distributed variables with a normal distribution, descriptive statistics were mean ± standard deviation, and for non-normally distributed data, median (interquartile range). Frequencies and percentages were used to report categorical variables. For group comparisons, the Kruskal-Wallis test was used for non-normally distributed continuous variables, the χ2 or Fisher’s exact test was used for categorical data, and one-way ANOVA was used for continuous variables having a normal distribution. After using post hoc analysis and Bonferroni correction, the significant level was chosen at p < 0.016. The Levene test, which employs the Tukey test for homogeneously distributed significant parameters and Tamhane’s T2 test for non-homogeneously distributed significant parameters, was used to determine the homogeneity of normally distributed parameters. For continuous variables, Student’s t-test or the Mann-Whitney U test was used to compare two independent groups; for categorical data, the χ2 or Fisher’s exact test was employed. P-values less than 0.05 were deemed statistically significant. For continuous variables having a normal distribution, we used the Pearson correlation coefficient; for variables with a non-normal distribution, we used Spearman’s rank correlation coefficient. By using ROC curve analysis, the optimal cut-off value for the average PFT to predict the outcome of CTO-PCI was found. Independent mortality predictors were found using Cox regression analysis; the results are shown as hazard ratios (HRs) and 95% confidence intervals (CIs). The Kaplan-Meier method was used for survival analysis, and log-rank tests were used to evaluate variations in survival parameters.
Results
The study included 415 patients, with a mean age of 62.8 ±10.2 years, and 30.6% were female. Initially, patients were stratified into three groups based on CAG results. The medically managed group was classified as ‘normal,’ the non-CTO-PCI group as the ‘PCI (non-CTO) group,’ and patients with chronic total occlusions (CTO) who underwent PCI as the ‘CTO-PCI group.’ Table I summarizes these three groups’ demographic, clinical, and CT coronary angiogram characteristics. No significant differences were noted in gender, age, smoking, or chronic obstructive pulmonary disease among the groups. However, notable differences were observed in diabetes mellitus (19 (13.6) vs. 33 (23.7) vs. 47 (34.6), p < 0.001), hypertension (49 (35) vs. 63 (45.3) vs. 68 (50), p = 0.036), dyslipidemia (16 (11.4) vs. 22 (15.8) vs. 42 (30.9), p < 0.001), left ventricle ejection fraction (LVEF) (61 (55–67) vs. 60 (30–65) vs. 55 (20–65), p < 0.001), fasting blood glucose (101 (73–318) vs. 107 (74–359) vs. 116 (69–564), p < 0.001), creatinine (0.9 (0.5–1.5) vs. 0.9 (0.6–1.9) vs. 1 (0.7–5.7), p < 0.001), high-density lipoprotein cholesterol (HDL-C) (45 (25–81) vs. 42 (25–86) vs. 40 (18–98), p < 0.001), hemoglobin (13.8 ±1.6 vs. 13.4 ±1.7 vs. 12.7 ±2, p < 0.001), C-reactive protein (CRP) (2 (0.1–17.5) vs. 2.5 (0.1–15) vs. 3.5 (0.1–104), p < 0.001), albumin (4.1 ±0.2 vs. 4.1 ±0.3 vs. 3.9 ±0.3, p < 0.001), LAD-PFT (6.4 ±1.7 vs. 7.1 ±1.6 vs. 6.9 ±1.8, p = 0.001), LCx-PFT (12.4 ±2.1 vs. 13.4 ±2 vs. 15.1 ±1.8, p < 0.001), RCA-PFT (14.8 ±2.5 vs. 16.3 ±2.6 vs. 18.3 ±3.2, p < 0.001), and average PFT (11.2 ±1.6 vs. 12.3 ±1.5 vs. 13.4 ±1.7, p < 0.001) among the groups.
Table I
Baseline characteristics of patients according to the coronary angiogram result
[i] PCI – percutaneous coronary intervention, CTO-PCI – chronic total occlusion percutaneous coronary intervention, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, CRP – C-reactive protein, LAD-PFT – left anterior descending artery pericoronary fat thickness, LCx-PFT – left circumflex artery pericoronary fat thickness, RCA-PFT – right coronary artery pericoronary fat thickness.
Post hoc analysis assessed significant differences between the groups, with Bonferroni correction applied to adjust the new p-values, setting the significance limit at 0.016. Regarding hemoglobin levels, a significant difference was observed only between the normal group and the CTO-PCI group (p < 0.001), with no significant differences between the CTO-PCI and PCI (non-CTO) group or between the PCI (non-CTO) and normal group (p = 0.022 and p = 0.123, respectively). In terms of albumin levels, significant differences were noted between the CTO-PCI and normal group, as well as between the CTO-PCI and normal group (p < 0.001 for both), while no significant difference was found between the normal and PCI (non-CTO) groups (p = 0.737). For LAD-PFT, significant differences were observed only between the PCI (non-CTO) and normal group (p = 0.001), but not between the CTO-PCI and PCI (non-CTO) groups (p = 0.580), and normal and CTO-PCI groups (p = 0.028). Significant differences were found in LCx-PFT and RCA-PFT between all groups (p < 0.001 for all comparisons). Regarding average PFT, significant differences were observed between the CTO-PCI and PCI (non-CTO) groups, between the CTO-PCI and normal groups, and between the PCI (non-CTO) and normal groups (p < 0.001 for all comparisons).
This study further stratified patients into two groups based on their survival status. Over a median follow-up period of 16.6 ±10.3 months, the CTO-PCI group exhibited a mortality rate of 13.9%. Table II summarizes these study groups’ demographic, clinical, and CT coronary angiogram characteristics. Notably, no significant differences were observed in gender, age, smoking status, presence of diabetes mellitus, hypertension, dyslipidemia, chronic obstructive pulmonary disease, history of unsuccessful procedures, prior CABG, or the target CTO vessel between the two groups. However, the non-survival group displayed distinct characteristics compared to the survival group. Specifically, individuals in the non-survival group had a significantly lower LVEF (42 ±13 vs. 53 ±10, p < 0.001) and higher scores on the J-CTO score (1.9 ±0.9 vs. 1.3 ±1, p = 0.015), EuroCTO (CASTLE) score (2.9 ±0.9 vs. 1.7 ±0.9, p < 0.001), and PROGRESS-CTO score (1.8 ±1.4 vs. 1.1 ±1, p = 0.048). Laboratory parameters further distinguished the non-survival group, revealing significantly lower levels of hemoglobin and albumin compared to the survival group. In terms of CT coronary angiogram evaluation, the non-survival group exhibited higher values for RCA-PFT (21.2 ±4.1 vs. 17.7 ±2.7, p = 0.002) and average PFT (14.5 ±2 vs. 13.2 ±1.5, p = 0.013).
Table II
Baseline characteristics of CTO-PCI patients according to survival status
[i] CTO-PCI – chronic total occlusion percutaneous coronary intervention, CABG – coronary artery bypass graft surgery, J-CTO – Multicenter Chronic Total Occlusion Registry of Japan, CASTLE – Coronary artery bypass graft history, Age (≥ 70 years), Stump anatomy (blunt or invisible), Tortuosity degree (severe or unseen), Length of occlusion (≥ 20 mm), and Extent of calcification (severe), PROGRESS-CTO – Prospective Global Registry for the Study of Chronic Total Occlusion Intervention, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, CRP – C-reactive protein, LAD-PFT – left anterior descending artery pericoronary fat thickness, LCx-PFT – left circumflex artery pericoronary fat thickness, RCA-PFT – right coronary artery pericoronary fat thickness.
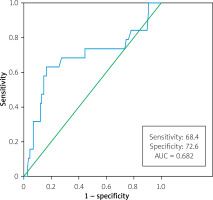
A receiver operating characteristic (ROC) curve analysis was performed to predict mortality, yielding an average PFT cut-off value of 13.6 mm, with a sensitivity of 68.4% and specificity of 72.6% (AUC = 0.682, 95% CI: 0.528–0.835, p = 0.011) (Figure 2). Table III provides a comprehensive summary of the study groups’ demographic, clinical, and CT coronary angiogram characteristics based on average PFT (average PFT ≥ 13.6 mm vs. average PFT < 13.6 mm). Notably, the group with higher average PFT demonstrated significantly higher J-CTO score and total cholesterol level, and lower hemoglobin levels.
Table III
Baseline characteristics of patients based on average pericoronary fat thickness cut-off
[i] PFT – pericoronary fat thickness, CABG – coronary artery bypass graft surgery, CTO – chronic total occlusion, LAD – left anterior descending artery, LCx – left circumflex artery, RCA – right coronary artery, J-CTO – Multicenter Chronic Total Occlusion Registry of Japan, CASTLE – Coronary artery bypass graft history, Age (≥ 70 years), Stump anatomy (blunt or invisible), Tortuosity degree (severe or unseen), Length of occlusion (≥ 20 mm), and Extent of calcification (severe), PROGRESS-CTO – Prospective Global Registry for the Study of Chronic Total Occlusion Intervention, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, CRP – C-reactive protein.
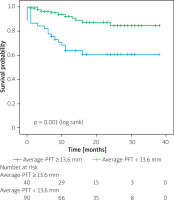
Table IV summarizes the results of both univariable and multivariable Cox regression analyses for mortality-related factors. Additionally, Kaplan-Meier analysis was performed to evaluate outcomes based on average PFT. The table highlights significant associations with mortality for LVEF (HR = 0.938; 95% CI: 0.901–0.975; p = 0.001), albumin (HR = 0.189; 95% CI: 0.058–0.617; p = 0.006), and average PFT (HR = 1.252; 95% CI: 1.010–1.552; p = 0.040). Moreover, Kaplan-Meier curves demonstrate notable differences in mortality associated with an elevated average PFT (p = 0.001) (Figure 3).
Table IV
Independent predictors of mortality in Cox regression analysis and the effect of average PFT
Discussion
The primary objective of our study was to investigate the correlation between PFT and long-term mortality following CTO-PCI. Our results revealed a significant association between elevated average PFT, as observed and easily measured in CT coronary angiograms, and increased mortality. This underscores the potential utility of PFT as a prognostic marker in patients undergoing CTO-PCI.
Chronic total occlusion (CTO) PCI has traditionally faced intense scrutiny due to its greater procedural complexity, lower success rates, and higher complication rates compared to non-CTO-PCI [6]. At the same time, significant progress has been made over the last decade to enhance success rates and safety margins. However, the factors influencing the long-term prognosis of CTO-PCI remain unclear, with limited research in this area. Several observational studies have previously noted a link between unsuccessful CTO-PCI and poorer long-term clinical outcomes, including elevated mortality rates [22–24]. The worse outcomes in the unsuccessful group could be attributed to more severe coronary heart disease or the potential adverse effects resulting from the intervention attempts. However, some studies have indicated that successful CTO-PCI, compared to failed PCI, does not necessarily translate to a reduced mortality risk [25–27]. It is important to note that many of these findings in the literature are derived from observational studies, which may introduce confounding factors. In our study we observed higher all-cause mortality in patients with unsuccessful CTO-PCI, although this difference did not reach statistical significance. This uncertain result underscores the complex interplay of factors influencing the long-term prognosis in CTO-PCI and highlights the need for further research to understand better these dynamics and potential determinants of patient outcomes in this specific context. Furthermore, acknowledging that the full scope of the benefits from successful CTO-PCI cannot be adequately understood by merely comparing outcomes of successful versus failed procedures underscores the need for randomized comparative studies.
Adipose tissue, distributed throughout the human body, serves as an energy storage site and provides insulation to tissues and organs. Over the past decades, its pivotal role in endocrine signaling has gained recognition. Adipose tissue exists in diverse forms, encompassing white, brown, and beige fat, and can be classified into subcutaneous adipose tissue and visceral adipose tissue (VAT), distinguishing them by their metabolic properties and anatomical locations [28]. VAT, a hormonally active component, produces molecules and hormones that impact normal and pathological processes, both locally and systematically. In conditions such as obesity, an accumulation of VAT, including epicardial adipose tissue (EAT) located between the myocardium and the visceral layer of the pericardium and covering 80% of the cardiac surface, may contribute to comorbidities such as diabetes and atherosclerosis [29]. EAT, in direct contact with the myocardium, releases factors with a paracrine effect on cardiomyocytes and is implicated in CAD development [30]. Studies suggest that dysfunctional EAT is associated with coronary inflammation and plaque severity, emphasizing its role in plaque vulnerability [31]. Although standardized recommendations for measuring EAT are lacking, PFT is increasingly recognized as a specific imaging marker for coronary inflammation. PFT specifically denotes adipose tissue within the EAT depot surrounding coronary arteries, closely interacting with adjacent coronary arteries [16, 17]. Pericoronary fat, the adipose tissue surrounding coronary arteries, is metabolically active and can produce pro-inflammatory cytokines that exacerbate local inflammation. Increased PFT is associated with higher levels of local inflammation, which contributes to the progression and instability of atherosclerotic plaques. This relationship is supported by imaging studies showing that greater PFT correlates with markers of coronary inflammation. Studies have shown that increased PFT is associated with a higher risk of major adverse cardiovascular events (MACE) and mortality. For instance, research by Mahabadi et al. demonstrated that higher epicardial fat volume, which includes pericoronary fat, is linked to an increased risk of coronary artery disease and mortality, independent of traditional risk factors [32]. Similarly, a study by Goeller et al. found that PFT, measured by coronary computed tomography angiography (CCTA), predicts the progression of coronary artery disease and adverse cardiac events [10].
Thickness, volume, and attenuation are the three measurement techniques that have been used in the literature for the CT evaluation of PFT [33]. One often used metric for PFT measurement is the thickness of the adipose tissue surrounding the coronary artery. In ordinary clinical practice, the maximal width of EAT around the proximal coronary artery is often estimated using cross-sectional CT images, which is a simple and effective approach. Elevated EAT thickness was described in CAD by Xie et al., with a substantial increase noted as CAD severity increased [34]. In 80 patients evaluated by CT coronary angiography, Gać et al. found a positive connection between severe CAD, EAT thickness, and PFT [35]. Several studies have consistently shown a correlation between EAT thickness, PFT, and obstructive CAD [36, 37]. These investigations commonly utilized CT coronary angiography as the primary imaging technique. Although various methods have been employed for both EAT and PFT measurements in CT coronary angiography, direct measurement of adipose tissue around all three coronary arteries has been frequently utilized [35–37]. Our study adopted a similar approach. We measured average PFT and analyzed the average thickness of the pericoronary fat around the three coronary arteries, exploring its association with survival after CTO-PCI.
Ultimately, the parameters to predict prognosis after CTO-PCI have not yet come to light. In light of the current literature, no explicit parameter has been found to determine prognosis other than successful CTO-PCI. Our hypothesis is grounded in the fact that inflammation is a pivotal factor in determining the prognosis after CTO-PCI. Therefore, we sought to assess whether the measurement of PFT, considered a crucial marker of coronary inflammation, through CT coronary angiography, could serve as a determinant of prognosis following CTO-PCI.
Limitations of the study. It is important to acknowledge some inherent limitations of our research. First, there may have been selection bias due to the retrospective methodology and the small sample size, which limited the findings’ wider applicability. Our findings need to be confirmed and expanded upon in larger prospective cohort studies in the future. Secondly, while direct measurement of pericoronary fat is straightforward and feasible, employing digital software for a more detailed assessment of EAT volume could offer enhanced insights into inflammation. Thirdly, the follow-up period has a quite wide spread. Lastly, we calculated PFT using manual measurements; however, we reduced the possibility of bias by following a strict protocol and regularly using digital zoom to improve measurement accuracy.
Conclusions
Our study underscores the significance of coronary artery inflammation in predicting the prognosis of CTO-PCI. The measurement of PFT through CT coronary angiography has emerged as a rapid, simple, and easily applicable method in routine clinical practice. Particularly, the assessment of average PFT holds promise as an additional diagnostic tool for determining CTO-PCI prognosis.
A definitive parameter to ascertain the long-term prognosis of CTO-PCI remains elusive, with limited studies addressing this aspect in the existing literature. While the association of inflammation with coronary artery disease is well established, its impact on outcomes following CTO-PCI remains largely unexplored. In our study, we ventured into uncharted territory by investigating, for the first time in the literature, the influence of PFT, an inflammation marker, on the prognosis of CTO-PCI. Nevertheless, more investigation is necessary to confirm and clarify the molecular basis of these correlations.