Introduction
The lipid hypothesis of atherogenesis regards atherosclerosis as a lipid storage disease that begins with subendothelial build-up of low-density lipoprotein cholesterol (LDL-C) [1]. Nevertheless, this is only a hypothesis, and the etiology of atherosclerosis has evolved over a span of time. To date, etiological research has identified numerous risk factors for atherosclerosis, and many believe that high cholesterol is one of the most important.
Literally, a causative factor cannot be a risk factor at the same time. This means no more and no less that hypercholesterolemia cannot be the cause, simply because it is already a well-known risk factor for atherosclerosis with a minor impact. In support of this assertion, for instance, no or a very weak association was found between LDL-C concentration and coronary calcification and the severity of coronary artery disease (CAD) imaging by cardiac computed tomography angiography (CCTA) [2–4]. In addition, LDL-C does not correlate with the degree of coronary atherosclerosis in invasive angiography and with peripheral atherosclerosis [5, 6]. There is even the concept of the cholesterol paradox [7]. For example, the analysis of serum lipids among 7,744 patients undergoing coronary artery calcium (CAC) or CCTA scanning revealed that LDL-C was inversely related to all-cause mortality [8].
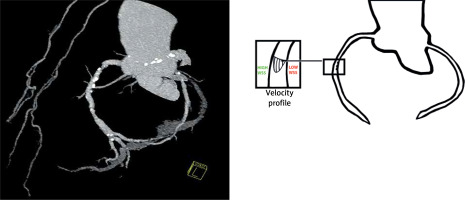
Nevertheless, the lipid hypothesis of atherogenesis does not explain why hypercholesterolemia spares some arteries, such as the interior mammary arteries, while other sites within the vasculature commonly harbor lesions [9] (Figure 1). Therefore, the orthodox connotation of alterations in lipid metabolism with atherosclerosis requires verification.
Figure 1
On the left side, angio view of the right and left coronary arteries. Despite advanced atheromatous lesions in the coronary arteries, both internal mammary arteries are free of atherosclerosis. On the right side, a schematic diagram of the velocity profile in the lumen of a curved coronary artery. Atheromatous lesions were more common on the myocardial side than on the opposite epicardial side of the arteries
In this review, we focus on the atherosclerotic lesion topography in the coronary arteries in autopsy studies and imaging modalities. In addition, alternative explanations for the non-random distribution of lesions based on hemodynamic disturbances and their effect on the vessel wall are discussed [10–13].
Natural history of atherosclerosis and normal atheromatous plaque distribution in coronary arteries – autopsy studies
Atherosclerosis is a slow, progressive disease that may begin with the appearance of fatty streaks in the aorta [14]. Progression to fibrous plaques and more advanced lesions leads to symptomatic atherosclerosis [15]. Particularly susceptible sites are the aorta with its major branches, including lower extremity branches. An autopsy study of the coronary, carotid and superficial femoral arteries revealed that the initiation, speed of development, and phenotypic expression of atherosclerotic plaques are related to the anatomic localization of the arteries [16]. In addition, atherosclerotic lesions are not only site-specific, but occur predominantly on the outer walls of the bifurcation and on the inner curvatures [17].
The timing of atherosclerosis onset differs between individuals, but it has been proven that atherosclerosis begins in childhood as the deposition of cholesterol and its esters in the intima of large muscular arteries. The prevalence and extent of fatty streaks and fibrous plaques increase with age [18]. Moreover, it has been shown that the location and extent of coronary artery fatty streaks in young people correspond to the location and extent of raised lesions in the elderly [19, 20].
Several studies have shown that fatty streaks appear in the coronary arteries in the second decade of life [21]. However, Stary found that microscopic counterparts of fatty streaks are present in the left anterior descending (LAD) coronary artery in more than half of children 10–14 years of age [22]. The PDAY (Pathobiological Determinants of Atherosclerosis in Youth) research group revealed that approximately 1 in 20 men aged 25 to 29 years and 1 in 5 men aged 30 to 34 years had an advanced atherosclerotic lesion that caused stenosis of 40% or more in the proximal LAD artery from histological sections of LAD arteries from 760 victims of accidents, homicides, and suicides [23].
The particular LAD predisposition to plaque formation is confirmed by the comparison of post mortem computed tomography CAC study results with histologically observed calcification and the degree of luminal stenosis [24]. In addition, in the LAD and the left circumflex (LCx) artery lesions tend to gather in the proximal section, while in the right coronary artery (RCA) lesions have a more non-uniform distribution [25]. One study involving 102 hearts with CAD at autopsy demonstrated that proximal CAD was more common than distal CAD in both diabetic and nondiabetic patients [26]. Moreover, the most affected by spontaneous coronary dissection is the LAD, and vulnerable plaques leading to ST elevation myocardial infarction are found within the proximal third of the coronary arteries [27, 28].
The distribution of atherosclerotic plaques in CCTA
Calcification is the major feature of coronary atherosclerosis and occurs very early. However, it can only be detected when an increased amount is present, using either electron beam computed tomography or multidetector computed tomography. A positive calcium score emerges after the age of 40 in men and women [29]. Numerous CCTA studies have consistently reported the LAD susceptibility to atherosclerosis, less frequently the RCA, followed by the LCx and LM [30–35]. Similarly to post mortem examinations, CCTA studies also showed that proximal sections are atherosclerotic in the LAD and LCx, while the location of atherosclerotic plaques was more uniform in the RCA. Of the 30,154 participants in the SCAPIS (Swedish Cardiopulmonary Bioimage Study) cohort, in the early phase of atherosclerosis, defined as disease of only one coronary segment, the proximal LAD was most commonly involved [30]. In both patients with diabetes and renal diseases, the LAD was the most frequently involved vessel and the LM was the least affected [36, 37]. Using the CCTA methodology Bax et al. focusing on the extent and composition of atherosclerotic plaques among patients with varying risk of atherosclerotic CAD found that the atheromatous plaques in the LCx were decidedly less numerous and consisted of fewer non-calcified plaques, supporting prior evidence of a lower incidence of acute coronary events in this vessel [38].
A large prospective study evaluated 1,344 patients from an international registry after undergoing sequential CT scans with a mean interval of 3.3 years [32]. This study showed that plaque progression and the odds of an annual increase in plaque burden were less common with the LCx compared to the LAD and RCA. Researchers suggested that this is due to different stages of the disease process or different pathogenic milieu in the coronary arteries.
The EISNER (Early-Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) registry, focusing on patients with mild CAC, defined as a CAC score of 1-99, revealed that proximal CAC was associated with an increased incidence of major adverse cardiovascular events (MACE) [39]. Also, patients with proximal coronary involvement but without obstructive CAD had an increased risk of MACE in the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry [40].
The distribution of atherosclerotic plaques in invasive coronary angiography
Based on the data from the National Registry of Procedures of Invasive Cardiology study of 3,208 young (below 40 years of age) patients in 2014 and 2017, it was found that the most common infarct-related artery was the LAD [41].
Three-vessel intravascular ultrasound (IVUS) imaging in 392 patients found that plaque ruptures occurred predominantly in the proximal section of the LAD, proximal and distal sections of the RCA, and throughout the LCx [42]. Another IVUS analysis showed a gradient of LAD plaque involvement from proximal to distal with sparse disease in the distal LAD. In contrast to the LAD, the proximal-to-distal plaque gradient in the RCA was less distinct [43]. Halon et al. studied the detailed distribution of coronary lesions in 302 patients with CAD who underwent coronary angiography for chest pain. In the LAD, atherosclerotic lesions were most common around the first diagonal branch [44]. In the LCx, the lesions were clustered around the origin of the first marginal branch. In the RCA, there was a predilection for narrowing between the infundibular and acute marginal branches and specifically around the origin of the right ventricular branch [44]. According to the Cardiovascular Medicine Database of Saitama Sekishinkai Hospital, of 3,268 patients with chronic kidney disease who underwent percutaneous coronary intervention, coronary lesions were observed in LAD in 56% of patients, compared to 28% and 22% in the RCA and LCx, respectively [45].
Is there a reason for the non-random distribution of atherosclerosis in the coronary arteries? What is the local risk factor?
As mentioned above, although the entire vascular bed is constantly exposed to systemic risk factors, including hypercholesterolemia, the formation of atherosclerotic lesions in the coronary arteries is not uniform. This claim is confirmed by the following observations. First, plaques develop predominantly in the LAD, while the LCx artery is relatively spared. Second, the plaques in the LAD and LCx are present mainly in the proximal segments, while in the RCA the plaques are more evenly distributed. Third, the variation in the occurrence of lesions is almost always eccentric and develops predominantly at lesion-prone “risk points” of the arterial vasculature, such as the lateral wall of the coronary branching, particularly when the angle between the daughter branches is wide [46], or in the proximity to side branches such as septal and diagonal branches (Figure 2).
Figure 2
Curved MPR images of the left coronary artery bifurcation (A) and trifurcation (B). Calcified plaques are located on the lateral wall of the left anterior descending artery (A) and on the border of the lateral walls of the left main and left anterior descending artery (B). The anatomic features of the coronary division govern a complex local hemodynamic microenvironment (separation, recirculation, and secondary flow), leading to a local low and oscillatory wall shear stress, here located along the lateral walls of the vessel divisions. A schematic diagram of shear stress distribution in the left coronary artery trifurcation based on the principles of hemodynamics (C)
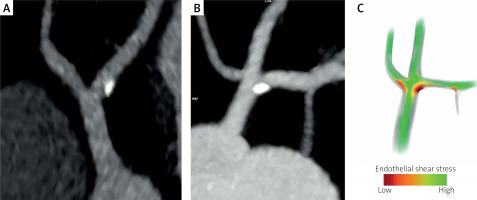
Therefore, there must be a reason for this striking feature of the topography. Several theories have been proposed to explain the specific location of atherosclerotic plaques, including the discontinuation of the internal elastic lamina or perivascular adipose tissue dysfunction [47]. However, recent evidence supports the hypothesis that the distribution of plaque results from the interaction of local hemodynamic forces (disturbed flow) within the vessel wall [48, 49]. Hemodynamic theory considers atherosclerosis to be a reactive biological response of endothelial cells (ECs) to the imposition of frictional mechanical drag of blood flow on the artery wall, called wall shear stress (WSS) [50]. ECs exposed to a disturbed flow with low WSS at the “risk points” undergo reorganization. Shear-induced alterations in ECs’ integrity and increased vascular permeability, with up-regulation of adhesion molecules, promote monocyte adhesion and migration into the subendothelial space. Further, elevated luminal LDL-C concentrations at low WSS locations contribute to LDL-C transport across the arterial intima [51–54]. Thus, both a low WSS and dyslipidemia promote an inflammatory response and plaque initiation and development.
As can be easily noted in the CCTA imaging technique, plaques develop along the inner curvature, where the blood flow is slower than along the outer wall. It is due to the non-axial blood flow inside the cross-section on the curved segments (the flow velocity in curved arteries is skewed to the outer wall, creating lower WSS on the inner curvature, where the chance of leukocytes’ and monocytes’ adhesion and LDL migration to the intima is greater) (Figure 3). Thus, the plaques grow circumferentially from the inner toward the outer curvature of the artery arch [55–57]. Therefore, local flow disturbances dictate an eccentric plaque nature [58].
Figure 3
Curved MPR images of left anterior descending artery – LAD (A, B), cinematic rendering of LAD (C), curved MPR image of cross-sections through the plaque (D), inverted image (E), color scale (F). An eccentric, partly calcified plaque is located next to the first septal branch – the milking-like effect of the septal perforator [70]. Positive arterial wall remodeling and preserved arterial lumen diameter during early atherosclerosis (Glagov’s remodeling) [56]
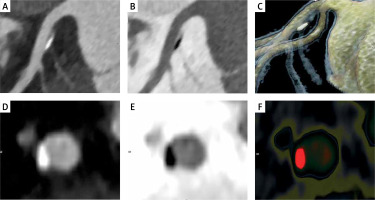
In vitro and in vivo experiments showed that ECs are critical sensors of shear stress that modulate endothelial structure, function, and permeability [59]. Low or low and oscillatory WSS triggers multiple pathways of intracellular signaling pathways that promote the expression of pro-proliferative and pro-inflammatory mechanosensitive genes while suppressing atheroprotective flow-dependent genes [60]. As a result, a flow-dependent inflammatory and infiltrative process is initiated with important implications for hemostasis and thrombosis, vascular tone, redox balance, vascular smooth cell proliferation and ultimately their osteoblastic transformation with subsequent plaque calcification [47]. This phenomenon of interplay of shear stress and ECs is called mechanotransduction and is a key mechanism linking blood flow to atherosclerosis [61–63]. Some authors postulate that local alterations in WSS may promote the transformation of stable plaque subtypes into unstable ones [64], and that a local low WSS provides incremental risk stratification of untreated coronary lesions in high-risk patients beyond the measures of plaque burden, low minimal lumen area and morphology [65].
These computational fluid dynamics technologies, which enable the quantification of hemodynamic forces acting on plaques in patient-specific geometric conditions, may improve the identification of culprit lesions for future acute coronary syndrome [66].
One of the vital questions regarding atherosclerosis is whether the internal mammary artery and the radial artery in their normal anatomical position are resistant to atherosclerosis. Another is why the internal mammary artery is more durable when used as a coronary graft, whilst the radial artery used as an aortocoronary graft is no longer protected. A thought-provoking explanation for the second question may be that the internal mammary artery used as a by-pass encounters flow conditions in its native environment, while the radial artery is anastomosed to the aorta [67]. Therefore, it is not without reason that atherosclerosis is regarded as a type of flow-dependent disease, mainly of the aorta and its branches. The key factor for such atherosclerosis distribution may be the behavior of pressure and flow pulses in the arteries as they travel away from the heart toward the periphery [68].
Conclusions
Mechanical forces associated with complex blood flow play an important role in atherosclerosis. Disturbed laminar flow or turbulent flow reduces WSS, which in turn causes modification of ECs’ functions and structures with a subsequent flow-dependent ongoing inflammatory response. Increased vascular permeability and flow-dependent high luminal surface LDL-C concentration facilitate the LDL-C uptake in regions with low WSS [54, 69]. Shear-dependent upregulation of cellular adhesion molecules’ expression promotes the trapping of monocytes in the subendothelial space, maintaining the inflammatory response. Therefore, hypercholesterolemia and low WSS play in one orchestra, and long-term exposure of ECs to reduced WSS promotes the buildup of atheromatous plaques.
In line with the above comments, the propensity to form plaques in the coronary arteries should be related to the geometry of the vascular architecture. The favored proximal LAD plaques’ location should be attributed to the presence of side septal perforators and diagonal branches, which have a significant impact on the local flow field distribution and alter the local hemodynamic milieu [70]. The dispersion of atherosclerotic plaques in the LCx and RCA should also be ascribed to their branching pattern.








