Introduction
Different types of viruses have been designed to deliver genes to cells or destroy a spectrum of the neoplastic cells [1]. Adenovirus is an effective oncolytic virus and delivery vector with appealing characteristics which made it more popular for cancer gene therapy [2]. A major obstacle for efficient viral-based gene therapy and virus replication in the target tissue is the heavy induction of innate immunity, especially when employed systemically [1, 3]. Apart from the toxic inflammation and cytokine storm-related shock, adenovirus-mediated innate immunity is capable of mounting the adaptive immunity which could memorize the events later on. As a result, this immunological memory destroys virus-producing cells and limits re-administration of the viruses [4].
The understanding of innate immune induction mechanisms by adenoviruses (Ads) is a field of interest to control their natural toxicity. Following intravascular administration, Ads have been shown to form complexes with a variety of blood components, more importantly, coagulation factors [5]. These factors are harnessed by viral particles to target them into permissive host cells [6].
Pioneer studies showed that coagulation factors interacted with adenoviruses hexon protein to enhance the entry and transduction process when assessed in vitro [6, 7]. Coagulation factor X was initially determined to be a critical factor of tropism which facilitates the hepatocyte infection by adenovirus [7]. Of note, despite the high-affinity interaction of coagulation factor VII to hexon, supported evidence that its cooperation in the entry process on the hepatocyte is lacking. It is shown that in the case of human adenovirus type 5 HAd5, coagulation factor X is sufficient to mediate virus transduction on hepatocyte when evaluated in vivo [8]. However, any data regarding the importance of FVII in this process need to be investigated. In addition, it is demonstrated that a complex of adenovirus with coagulation factors induces different patterns of innate immunity when investigated in vivo and in vitro [9]. Several studies have been performed to describe the role of FX in innate immunity induction when it is loaded on adenovirus. Doronin et al. showed that binding of FX to the adenovirus which has accessed the blood circulation permits the sentinel immune cells to sense the FX-adenovirus complex through triggering the TLR4 signal pathway, and subsequently developed a stronger innate immunity [10]. In contrast, Xu et al. suggested that the decoration of adenovirus with FX molecules is an effective strategy to protect viral particles from antibodies and complement detection [11]. In a recent study, Duffy et al. showed that in the human sera, adenovirus induced complement activation less when FX was available for virus interaction [12].
In spite of different studies, questions about the role of coagulation factors in innate immune induction of adenovirus were mostly remained unanswered. It is unclear how much adenovirus-mediated immunity may be influenced by coagulation factors as they come together in the human body. Hence, comparing the contribution of coagulation factors such as FX and FVII in this process has not been considered, yet. The delineation of the extent of adenovirus immune induction by coagulation factors, especially in hepatocytes, could be beneficial in finding ways of inflammation control during gene therapy with these viruses. Herein, the immune induction potency of FVII or FX coated adenovirus was evaluated in the hepatocyte cell model.
Material and methods
Cell culture
Ad-293 (human embryonic kidney cell) and HepG2 cells, a human hepatocellular carcinoma cell line (HB-8065, ATCC), were purchased from Iranian Biological Resource Center (Tehran, Iran). The cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM) complemented with 10% fetal bovine serum (FBS), with 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. The cells were distributed in 6-well plates in 0.3 × 106 cells/well and treatment was performed when their confluence reached 80%. They were incubated under humidified standard conditions, at 37°C and 5% CO2. Ad-293 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented as above.
Virus propagation and titration
Human adenovirus 36 (ATCC VR1610) was a kind gift from Dr. Ali Teimouri (Hamadan University of Medical Sciences). It was selected based on the similar attachment and entry process of common oncolytic adenoviruses which is CAR-dependent. A recombinant adenovector type 5 expressing green fluorescent protein (GFP) (Ad5-GFP) was also prepared in our laboratory, formerly [13]. In order to amplify these viruses, Ad-293 cells were seeded in T175 culture flasks; then, the rounded cells containing viruses were harvested, washed with phosphate buffer (PBS), and freeze-thawed to release the viruses. The concentrated viruses were then prepared from supernatant of destroyed cells following 2 rounds of 4000 rpm centrifuge, passing through 0.4 µm size filter and Amicon Ultra-15 Centrifugal Filter Devices (EMD Millipore Corporation, Billerica, MA, USA). Then, the prepared viruses were stored with 10% (V/V) glycerol at –70°C until further experiments.
In order to determine the viral titer, we employed 50% tissue infective dose assay (TCID50). The endpoint was taken to be the highest dilution of the virus that produced a cytopathic effect (CPE) in 50% of the inoculated cells [14]. TCID50 titer was converted to the number of infectious units per volume, and then divided by the number of cells to be infected in order to obtain the suitable MOI (multiplicity of infection) of viruses.
In addition, to estimate the suitable effective and non-cytotoxic MOI of the virus, the trypan blue exclusion test was performed. To do this, virus of different MOI ranging from 0.01 to 10 MOI was added into the HepG2 cells and incubated for 24 hours at 37°C. Afterward, the cells were harvested, then stained with trypan blue and evaluated under light microscopy. The percentage of live cells was considered in selecting the suitable dose of inoculation.
Zeta potential and particle size distribution
Particle size and zeta potential of the adenovirus-coagulation factor complexes were measured after 1 hour at 37°C of incubation, using a NANO-flex – 180° DLS and ZETA-check (Microtrac, Meerbusch, Germany). The viscosity and refractive index of pure water at 25°C were used for data analysis. The intensity-average hydrodynamic size (Z-average) was determined at the suitable concentration. Zeta potential measurements were performed in phosphate-buffered saline (pH = 7.4) using the calibrated streaming potential method.
Virus-coagulation factor complexes preparation and Hep-G2 infection
Recombinant human blood coagulation factors zymogens VII and X were purchased from Hematologic Technologies, Inc. (Essex Junction, VT, USA). They were reconstituted in PBS and then stored at –80°C until further processes. Adenovirus was diluted in serum-free DMEM to reach 0.5 MOI, and then the coagulation factors were added at physiological concentration. They were then incubated for 1 hour at 37°C until the virus/factor complex formed. About 10 µg/ml and 0.6 µg/ml of FX and VII proteins corresponding to their physiological concentration in the blood (1 U/ml) were included in each tube, respectively [6]. The Hep-G2 cells were seeded into 6-well plates one day prior to infection, while they were divided into 4 cell groups receiving Ad36 (AD), Ad36-FX complex (AD-X), Ad36-FVII complex (AD-VII), or medium (Cell), respectively. For infection, the cells were washed twice with PBS then virus/coagulation factor complexes in a serum-free DMEM were added per well and incubated for 6 more hours at 37°C.
Transduction efficiency
In order to evaluate the virus transduction efficiency on Hep-G2 cells, different MOIs of recombinant Ad5-GFP were tested alone or in complex with coagulation factors (Ad5-GFP, Ad5-GFP-FVII and Ad5-GFP-FX). MOI = 1 was selected for further evaluation. In the 48-well plate, different complexes of Ad5-GFP vector were inoculated at MOI = 1 following 1-2 hours absorbance. Afterwards, the infected cells were fed with 2% complete medium, and then they were left in CO2 incubation atmosphere for 24 hours [8]. The transduced cells were finally estimated by fluorescent microscopy through evaluating the green fluorescence from cells, indicating GFP expression as well as adenovector presence. To assess the percentage of positive cells, GFP signals were simply counted on each field whilst light and fluorescent images were overlaid, then the mean of repeated tests was considered as the estimated percentage. Also the detailed quantification of GFP positive cells was performed by flow cytometric analysis using a BD FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The data were analyzed with FlowJo software.
Real-time PCR analysis
Total cellular RNA was isolated from normal HepG2 as a control and treated HepG2 (treated with AD, AD-VII, and AD-X) with an RNA extraction kit (CinnaGen Inc., Tehran, Iran). The quantity and quality of the obtained RNA were checked by measuring the ratio of optical density of 260/280 nm, using a Nanodrop spectrophotometer (Nanodrop; Thermo Fisher Scientific, Wilmington, DE, USA), and then it was stored at –80°C until cDNA synthesis. The cDNA was then synthesized using 1,000 ng of total RNA in a first-strand cDNA synthesis reaction using the PrimeScript RT reagent Kit (Perfect Real Time) (TaKaRa Bio, Siga, Japan). Quantitative polymerase chain reaction (qPCR) was performed using the ABI Biosystems 7500 and the RealQ Plus 2× Master Mix Green (Ampliqon A/S, Odense, Denmark). In each reaction, 100 nM of each primer was added to target the specific sequence. Specific primers targeting IL-1β, IL-8, IL-18, and protein kinase R (PKR) were designed, as shown in Table 1. Primer pairs were designed using Allel ID software (Applied Biosystems). The PGK housekeeping gene was also used as the internal control of qPCR normalization. The qPCR conditions were set for 15 min at 95°C followed by 40 cycles of 15 s at 95°C, 30 s at 54-58°C and final extension of 30 s at 72°C. The amplification signals of different samples were normalized to the PGK cycle threshold (Ct), and then the 2-ΔΔCT method was applied for comparing the mRNA expression levels of groups against the control, normal HepG2, which was represented as fold change in data analysis.
Table 1
Primers used in the study
Statistical analysis
All experiments were performed a minimum of three times. Data were expressed as the mean ± standard error of the mean and analyzed using one-way ANOVA or the Kruskal-Wallis test using GraphPad Prism 7.03 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons of the groups for determinations of statistical differences were done using Student’s t-test. P < 0.05 was considered to indicate a statistically significant difference between the group means.
Results
Cytotoxic effects of adenovirus on HepG2
In order to investigate the cytotoxic effect of adenovirus on HepG2, adenovirus of different MOIs was added into the cell culture. The light microscopy and cell staining showed that at MOI > 1 a portion of cells started to detach and acquire a necrotic shape (> 50%), which indicated significant cytotoxicity of adenovirus in those doses. This finding also revealed that MOI = 0.5-1 as well as the incubation time of 6-12 hours could be effective as well as non-toxic for further immune response evaluation.
Transduction assays of Ad5-GFP in Hep-G2
To assess whether loading coagulation factor VII and X on adenovector increase its transduction efficiency, fluorescent microscopy and flow cytometry were employed. Interestingly, GFP fluorescent numeration showed that different adenovector complexes could reach the nucleus, efficiently. Moreover, microscopic evaluation showed that loading with coagulation factors enhanced the transduction rate of Ad5-GFP as Ad5-GFP-FX (60%) and Ad5-GFP-FVII (50%) exhibited an increase in transduction potency (p < 0.05) in comparison to Ad-GFP (35%) (Fig. 1A). In addition, flow cytometry results were consistent with fluorescent microscopy and indicated the superiority of FX over VII for boosting the transduction efficiency of Ad5-GFP in Hep-G2 (p < 0.05), as shown in Fig. 1B.
Particle size and Zeta Potential
Volume-weighted distribution of AD after incubation with FVII and FX changes from unimodal to multimodal pattern. Similarly, multiple peaks are observed in light intensity-weighted distribution with an increase in average hydrodynamic size following coagulation factor loading on AD. Furthermore, zeta potential of the viral particle (–27 mV) undergoes subsequent changes by coagulation factors (data not shown). These findings demonstrate that loading of coagulation factors on the virus leads to an increase in the mean size and absolute zeta potential to different extents.
Pattern of innate immune gene, PKR expression
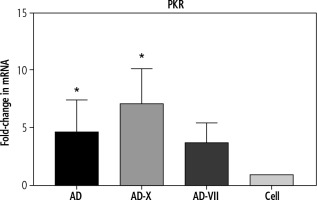
In order to evaluate the innate immune induction, PKR was measured by qPCR. The results showed that all types of viruses were able to induce PKR gene expression. These findings demonstrated that the adenovirus-FX complex (AD-X) exhibited the most significant increase in PKR expression level (p = 0.0164), followed by a similar level for AD (p = 0.0152) and adenovirus-FVII (AD-VII) (4.6-, 7.1-, and 3.7-fold, respectively), when compared to the control cell (Fig. 2).
Fig. 2
The effect of different treatments on PKR gene expression of Hep-G2. The results, which are represented as fold changes in mRNA level in the cells treated with AD, AD-X, and AD-VII relative to the control cells, are means ± SE (bars). *p < 0.05 compared with the control cells at the same point. AD – adenovirus 36, AD-X – adenovirus 36-FX, AD-VII – adenovirus 36-FVII
Pattern of IL-1β, IL-8 and IL-18 gene expression
The pattern of IL-1β expression revealed that following treatment with AD, AD-X, and AD-VII, its level increased up to 2.7-, 5.1- and 2.0-fold compared with normal cells, respectively. These findings demonstrated that the AD-X virus complex exhibited the most significant increase in the IL-1β level (p = 0.0041), followed by AD and AD-VII, when compared to the control cell. However, there was no statistically significant difference between adenovirus groups (Fig. 3A).
Fig. 3
Effect of different complexes on inflammatory gene expression of Hep-G2. The results, which are expressed as fold changes in mRNA level in the cells treated with AD, AD-X, and AD-VII relative to the respective control cells, are means SE (bars). *p < 0.05, **p < 0.01, compared with the control cells at the same point
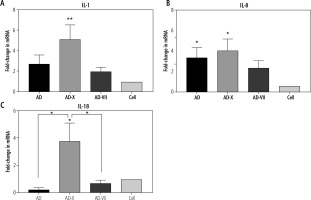
For IL-8, it was shown that Hep-G2 infection by AD, AD-X, and AD-VII groups induced an increased expression up to 6.3-, 7.5-, and 4.3-fold when compared to the normal group, respectively. The AD-X virus complex showed a significant rise in the IL-8 level (p = 0.0107), followed by a similar level for AD (p = 0.0370) and AD-VII (p > 0.05). However, there was no statistically significant difference between these groups (Fig. 3B).
In contrast, IL-18 has no significant fold difference in AD and AD-VII treatment groups relative to the normal group, but instead rose up to 3.7-fold in the AD-X infection group (p = 0.0193). In this case, AD-X showed a significant increase even when compared with other adenovirus groups including AD (p = 0.0360) and AD-VII complex (p = 0.0122) (Fig. 3C).
Discussion
The systemic administration of adenovirus induces innate toxicity following viral components’ sensing by different cells [4, 15]. Recent investigations suggested that coagulation factors, particularly FX, cause the virus to be targeted in the liver and infect the hepatocytes through a process that relies on the hexon interaction [6, 16]. Regarding the importance of innate toxicity, understanding the mechanisms and responsible molecules would be beneficial in finding new strategies to decrease inflammation and to reduce the burden of systemic administration [17]. In previous studies, the role of coagulation factors, especially FX, in increasing the efficiency of transduction in the epithelial and macrophage cells, as well as their effect on innate immune induction, has been suggested in vivo and in vitro [18-21]. Despite a study on the effect of FX [20], there are limited data about the role of other coagulation factors in the innate immunity process of the most important cells during systemic injection – the hepatocytes [18]. Moreover, a comparison between FVII and FX (important coagulation factors which interact with adenovirus) has not been performed yet to determine the extent of innate immune induction in the hepatocytes [6]. Herein, the immune-inflammatory response of adenovirus type 36 (AD) was compared with Ad36 coated with FX (AD-X) and FVII (AD-VII) complexes on Hep-G2 cell line.
To determine the transduction rate between these groups, first we investigated the transduction efficiency of Ad5-GFP loading with FVII or FX. The GFP signal detection under fluorescent microscopy and flow cytometry showed that FX and FVII could increase the rate of entry [8]. The more detailed data of flow cytometry also showed that the FX can enhance the virus transduction rate up to 72%. A comparison of the role of FX and FVII in transduction efficiency of adenovector was reported for the first time.
It has been shown that FX simply binds to the Ad hexon via charge-dependent interaction through hypervariable regions of the hexon [5]. In our study, Zetasizer evaluation demonstrated that simple mixing of the Ad36 and blood coagulation factors (both FVII and FX) increased the average particle size as well as the negative zeta potential charge, demonstrating the true loading of coagulation factor on particles.
The immune gene expression results indicated that the FX enhanced the innate immunity and inflammation caused by the Ad36 contrary to the FVII factor. Regarding the qPCR, the expression pattern of the PKR gene as an innate immunity marker revealed the significant role of FX. However, sole Ad36 and AD-VII complex induced PKR expression in a similar pattern. It is necessary to investigate whether this result is a consequence of the virus entry into the hepatocyte or due to viral interaction with an undefined molecular sensor in the host. Similar to this study, Dronin et al. in 2012 showed that adenovirus coated with FX induced stronger immune inflammation when compared with the virus alone, in the mice macrophage [10]. In spite of this, another study on human macrophage and dendritic cells (DCs) showed that Ad5-FX was not capable of activating TLR/NF-кB-related innate immunity in the mononuclear phagocytes or induced proinflammatory cytokine [20]. In addition, adenovirus complexes with FX and FVII were observed to attach and enter into the hepatocyte with the help of heparan sulfate proteoglycan (HSP), so it seems that the entrance pathway does not influence the inflammatory response [22-25].
The expression patterns of IL-1β, IL-8, and IL-18 were also in accordance with the PKR result as all adenovirus treatments showed a significant degree of inflammation. Meanwhile, FX-coated virus exhibited the most significant inflammatory effect on the Hep-G2 cells. In previous studies, it has been shown that adenovirus is largely capable of inducing inflammatory immunity in many cells, especially the hepatocytes [26-29]. Infection of cells by human adenoviruses/vectors induces the expression of pro-inflammatory molecules following the sensing of the capsid, dsDNA and other viral components [29]. Activation of different TLRs (Toll-like receptors) and DNA sensors that trigger downstream signaling pathways activates the NF-kB and IRFs (IFN-regulatory factors) and ultimately switches on the innate immune toxicity [29]. The ability of the blood coagulation factors, particularly FX, to induce or reduce the innate and inflammatory immunity has been considered by others; however, the exact role needs more investigation [2, 10, 11]. It has been shown that FX may protect the adenovirus from the complement system attachment and ameliorate the innate immunity when becoming complex with FX, while Dornin et al. demonstrated an enhancing effect for FX in this purpose [10, 11]. They suggested that when FX binds to adenovirus, its structure might transform to a pathogen-associated molecular pattern (PAMP) to stimulate the innate immunity via the TLR/NF-кB pathway. Recently, the interaction of FX and FVII with surface glycoproteins of Herpesviruses has been demonstrated [30]. However, comparative reports about the innate toxicity of FVII versus FX molecules are limited.
In summary, irrespective of a similar rate of virus transduction enhancement, the results of this study for the first time suggested that coagulation factor X (FX) but not FVII has an inductive role in the adenovirus-mediated innate toxicity of hepatocytes. However, this study had some limitations including the lack of exact determination of the virus entrance steps by confocal microscopy or antagonists, absence of ELISA assay for cytokine measurement, and lack of any data about protein interactions with cell surface molecules. Another study suggests that an interaction between Ad-FX may result in a better attachment to TLR4, which will consequently enhance the systemic inflammatory response [20]. Hence, there are many factors which could be involved in this difference, but among them a closer interaction with cellular sensors seems to be more likely.







