Patients with coronary artery disease and low left ventricular ejection fraction (LVEF) undergoing coronary artery bypass grafting (CABG) have an increased risk of post-operative complications, especially sudden cardiac death (SCD) [1]. The waiting time for this group of patients to be eligible for implantation of a cardioverter-defibrillator (ICD) as primary prevention of SCD in Poland is 3 months after the patient is discharged from the hospital [2]. To protect these patients from SCD in the period between hospital discharge and ICD implantation, European Society of Cardiology (ESC) guidelines recommend the temporary use of a wearable cardioverter-defibrillator (WCD) [3, 4].
We present the first use of WCD LifeVest (ZOLL Pittsburgh, Pennsylvania, United States) in a patient with coronary artery disease and low left ventricular ejection fraction persisting after a CABG operation until ICD implantation.
A 63-year-old man with acute myocardial infarction, heart failure and diabetes was admitted to the Department of Cardiac Surgery Medical University in Lublin for surgical treatment. Coronary angiography showed borderline stenosis of the left coronary artery trunk, occlusion of the right coronary artery, anterior interventricular and circumflex artery, and critical stenosis of the diagonal and marginal branches. Echocardiography showed normal-sized heart cavities, reduced global left ventricular contractility with ejection fraction of 20%, mild mitral and tricuspid regurgitation and mild aortic stenosis. The patient was prepared for CABG surgery in an intensive cardiac care setting. Due to severe heart failure with continued EF of 20% on the evening before surgery, the patient received an intravenous infusion of levosimendan. Since there was no improvement of contractility, the patient was not eligible for immediate coronary artery bypass grafting at that time and was instead transferred to the Cardiology Department to undergo pharmacological preparation for surgery.
After 14 days, the patient was readmitted to the cardiac surgery department clinic, and after levosimendan administration CABG was performed. Due to advanced atherosclerotic lesions in the coronary arteries, only two peripheral anastomoses were made: to the anterior interventricular artery (left internal mammary artery – LIMA) and to the marginal branch (saphenous vein). After the surgery, the patient was mechanically ventilated with a continuous infusion of catecholamines and vasoactive drugs. After stabilization of the general condition, mechanical ventilation was terminated, and the patient was extubated. Following 3 days at the intensive care unit, the patient was transferred back to the Department of Cardiac Surgery. Infusion of the catecholamines dobutamine and epinephrine was maintained. Post-operative echocardiography showed a slightly enlarged left ventricle and a minimal improvement in LVEF (25%). In order to protect the patient from SCD for the time until ICD implantation, the patient was equipped with a WCD (LifeVest). The patient received take-home medical treatment (i.e., acetylsalicylic acid, clopidogrel, ivabradine, sacubitril/valsartan, metformin, metoprolol, torasemide, spironolactone, trimetazidine, atorvastatin) for further stabilizing treatment.
During re-evaluation of the patient at the Department of Cardiac Surgery, approximately 60 days after CABG, under constant echocardiographic control pleural drainage was performed without complications. 500 ml of straw liquid was obtained. The echocardiographic examination revealed no further global improvement of LVEF (25%) and a significantly reduced systolic function. Thus, the patient was found eligible for ICD implantation and was instructed to continue use of WCD after hospital discharge until ICD implantation was performed.
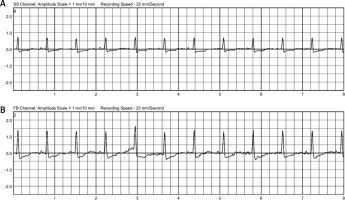
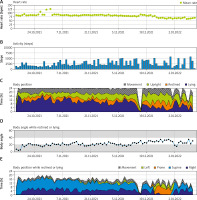
The integrated monitoring system of the WCD records parameters such as heart rate and rhythm, electrocardiography, wear time, activity, body position and detected arrhythmias, and transfers them to the ZOLL Patient Management Network (ZPM). The ZPM automatically processes and stores the data, which can be retrieved by the treating physician in order to evaluate whether adjustments to therapy and medication are needed. According to these data, the patient wore the WCD in total for 89 days almost constantly (94%) with an average daily use of 22.63 hours. The heart rate was on average 82 beats per minute (BPM). The patient walked daily on average 2,528 steps, spent most of the time reclining (40%) or lying (27%), supine (54%) or on the right side (33%) (Figure 1). During the entire wearing time, the WCD detected no life-threatening cardiac arrhythmias (Figure 2). No shock treatments were required, and no inappropriate shocks were delivered. The WCD use was discontinued because the ICD implantation was performed as planned 3 months after CABG.
Figure 1
Summary of heart rate, steps, body position and angle recorded by the wearable cardioverter defibrillator
The need for temporary protection from SCD by a WCD, especially in patients after cardiac surgery with an LVEF ≤ 35%, has been clearly pointed out by the ESC [3, 4]. This patient constantly had a severely reduced LVEF of ≤ 25% and was therefore at high risk for SCD. During the WCD use, no life-threatening cardiac arrhythmias were detected, and no inappropriate shocks were delivered. The WCD wearing compliance of the patient was very high, as the device was worn on almost all days (94%), with an average daily wearing time of nearly 23 hours.
In conclusion, the use of WCD for the first time in Poland in a patient with coronary artery disease and low LVEF after CABG surgery as protection against SCD for the period until ICD implantation confirms previous data on the safety of this therapy. These data confirm the benefits of using WCD to improve patient safety after cardiac surgery. The data were confirmed by a ZOLL representative. The VEST study and the HEARIT registry took place in Poland; in these studies there was no patient immediately after CABG, as in the present case report.