Introduction
The overall lifetime risk of fistula development in the course of Crohn’s disease (CD) ranges from 14% to 38% [1, 2]. Perianal fistula appearance can lead to a dramatic decrease in the quality of life in patients with CD, mainly due the appearance of specific symptoms, which include perianal pain, dyspareunia, dyschesia, and faecal incontinence, as well as complications such as abscess or perianal drainage. Perianal fistula development may precede for years or coincide with the first diagnosis of CD. The rate of fistula formation preceding diagnosis of CD can be as high as 45% [3, 4]. The rates of spontaneous fistula closure fluctuate between 6% and 19%, and importantly, despite the implementation of new therapeutic modalities such as anti-TNFs, the rate of success of combined medical and surgical treatment does not exceed 50% [1, 5, 6]. In recent years, significant progress has been made in perianal fistula management, which has evolved into a multidisciplinary approach that includes gastroenterologists, surgeons, and radiologists. Preoperative pelvic imaging for precise evaluation of the anatomy of the fistulous tract performed by an experienced radiologist in collaboration with a colorectal surgeon seems to be essential for choosing the best surgical strategy. Actually, the gold standard for fistula imaging in the course of CD is magnetic resonance imaging (MRI), which allows precise evaluation of morphology and complexity as well as relations to surrounding structures, including anal sphincters [7]. MRI is also considered the most reliable imaging method in the evaluation of an individual’s response to treatment [7, 8]. However, despite the significant progress in imaging techniques, including both contrast MRI and endoscopic ultrasound (EUS), proper understanding of perianal anatomy in CD patients remains challenging. A better understanding of the complexity of the relationships between fistula location and surrounding tissues potentially provides an positive input for surgeons in guiding clinical decisions, which may significantly improve outcomes.
The incidence of perianal fistulas other than perianal CD is relatively low; however, for each individual patient the degree of sophistication and complexity of the fistula is different. A study conducted by Sainio between 1969 and 1978 among Finnish citizens showed that the incidence of this problem was 5.5 cases per 100,000 women and 12.1 cases per 100,000 men [9]. Recent studies by Zanotti et al. at the beginning of the 21st century among populations of four European countries (Spain, England, Germany, and Italy) showed that the problem of anal fistulas affects from 1.04 to 2.32 people per 10,000 examined. Both studies carried out by Sainio and Zanotti et al. showed that twice as many men than women are struggling with the problem of perianal fistulas [8–10]. As shown in the above studies, perianal fistulas negatively influence a patient’s quality of life.
In addition to palpation examination, many techniques are available to identify and locate the fistula path and internal hole path [11]. Magnetic resonance imaging is the most precise radiological technique, and currently it is considered to be the gold standard for perianal fistula evaluation [12]. Images obtained by MRI allow the determination of the complexity of the fistula and its connections with the anal sphincter; therefore, a thorough understanding of the often sophisticated anatomy of the fistula is important for clinicians, and mutual cooperation between the radiologist and surgeon is of great importance. Three-dimensional (3D) technology is increasingly used in medicine [13, 14]. The continuous development of applications for 3D printing and the ever-lower price of the printout itself suggests that the future of this technology in medicine is promising. Anatomical objects printed in 3D enable the visualisation of complex cases allowing physicians, patients, and students to better understand the problem geometrically. Generating 3D models from DICOM (digital imaging and communications in medicine) images is possible when individual tissues have sufficient contrast to distinguish them. Selecting the thinnest layers during MRI examination significantly enhances the quality of modelling of the selected tissues [15–17].
Aim
Accurate characterisation of the fistula and identification of possible problems that may occur during treatment are extremely important for both the surgeon and the patient. Appropriate communication between the radiologist, surgeon, and patient is very important. In this manuscript, we describe a modern three-dimensional (3D) model based on MRI reconstruction of perianal fistula patients with CD.
Material and methods
MRI technique
MRI was performed using a 1.5 Tesla Optima MR360 Advance magnetic resonance imaging machine from General Electric. MRI was performed in 4 patients with a clearly marked perianal fistula. Each patient consented to participate in the study and for the use of MRI images in this article. Scans were made using a 16-channel TOTAL BODY coil. During the examination, the patient was in a supine position. A standard protocol for pelvis examination with minor modifications of the sequence parameters was used to obtain the images. The series of images were made using axial sequences (Axial T2 sequence parameters: MATRIX: 512 × 512, repetition time (TR) = 5854 ms, echo time (TE) = 102 ms, layer thickness = 2 mm, spacing = 0 mm, NEX = 2), sagittal (sequence parameters Sagital: MATRIX: 512 × 512, TR = 5316 ms, TE = 102 ms, layer thickness = 2 mm, spacing = 0 mm, NEX = 2), and coronal (Coronal T2 sequence parameters: MATRIX: 512 × 512, TR = 61 ms, TE = 102 ms, layer thickness = 3 mm, spacing = 0 mm, NEX = 3). A series of T2 images dependent on the above parameters and a series of images using the gradient sequence MERGE T2* were obtained (sequence parameters MERGE Axial T2*: MATRIX: 512 × 512, TR = 1189 ms, TE = 15 ms, layer thickness = 2 mm, spacing = 0 mm, NEX = 1).
3D modelling
After the MRI examination, the images in DICOM format were imported into 3D Slicer version 4.8.0 software. Using the images of the axial projection, segmentation was performed, and in the first stage the fistula locations were marked. For this purpose, manual drawing tools were used. The locations of the fistula on the images obtained from the MRI examination were marked by hand on each layer by a radiologist with the cooperation of medical physicist. The next step was to mark the places of the external anal sphincter and anal canal with a different colour. The last step was to mark the skin that was connected with the anus and the places of fistula outlet in the skin. The model thus prepared was then exported to a file in the Standard Tessellation Language (STL) format. Blender 2.77a software was used for the next stage of model creation, which allowed for smoothing of the edges of the created model and mitigation of the stairs effect (faults between successive layers/DICOM images).
3D printing
The next stage consisted of loading the model into the 3D Print program working with 3D ProJet 460Plus 3D printer. Before printing, specific colours were assigned to the individual elements of the model. The final version of the model contained from 700 to 1500 layers depending on the size of the model. The process of model creation and editing took about 2 h in total. The material used in the printing process was VisiJet PXL Core. The printing process took about 6 h.
Results
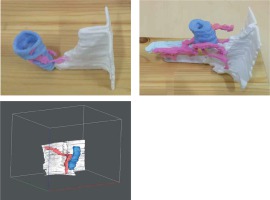
The images obtained from the MRI examination and the selected segment along with the model elements for one of the patients are presented in Figure 1. The individual structures of the gastrointestinal tract were marked with coloured markers by a radiologist (green – fistula tract; red – anal sphincter and anus; yellow – skin). The final version of the 3D model after smoothing all the edges was then exported to be sent to the 3D printer.
Figure 1
Selected layers from MRI examination for one of the patients. The individual structures of the gastrointestinal tract were marked with coloured markers by the radiologist (green: fistula tract; red: anus; blue: anal sphincter; yellow: skin)
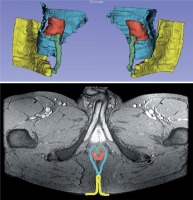
Segmented 2D MRI image presents an image of one of the layers with marked anatomical elements: green- fistula; red – anal sphincter and anus; yellow – skin, imported into the 3D Slicer 4.8.0 software. Axial MERGE MRI image presents anatomical image obtained from MRI study. At the bottom is a 3D model of a fistula duct made based on the above pictures (Figure 2).
Discussion
Many studies have confirmed that MRI is a gold standard for perianal CD visualisation [18]. Three-dimensional (3D) printing is a developing technique, which represents a significant technological achievement that was demonstrated to be useful in different biomedical applications. Therefore, combining both MRI and 3D printing techniques allows for accurate visualisation of the exact location of the anal fistula tract and its relationship with the surroundings, including anal muscle anatomy. It was shown in several studies that 3D printed models can precisely reveal the specific characteristics of the fistulae anatomy, and additionally the models have demonstrated potential in clinical practice [19–22]. Although our construction, when compared to all the above-mentioned studies, cannot be considered novel in terms of techniques applied or equipment used, our study provides a valuable contribution to the state-of-the art and shows that this technique could be applied elsewhere. Most studies describing the practical issues of 3D printing in GI surgery come from highly developed countries such as the UK, the USA, Canada, Japan, and Germany [23]. Because there is great emphasis on the use of evidence to support clinical intervention (as in NICE recommendations [24]), it is of great importance to provide more universal evidence.
Surgeons commonly visualise organs and structures with the use of 2D anatomical images. However, thanks to 3D technology, it is possible for the surgeon to see a real picture of the anatomy in question, and to problem solve prior to scalpel use. Moreover, accessibility to a 3D model before the start of a procedure will significantly reduce any unexpected circumstances during the course of surgery. Utilising this technique can also decrease the number of exploratory surgeries and associated procedures, decreasing damage to surrounding healthy tissues by more exactly defining the treatment area. A comparison of strengths and weaknesses of 2D and 3D techniques is presented in Table I.
Table I
Comparison of 2D and 3D technique
Continuing this pursuit, as others in this field [19–22], we believe that the practical application of the above-mentioned technique can be considered from three different viewpoints: technical assistance for surgeons, helping the patient in the decision-making process, and enhancing the education of medical professionals.
The main advantage for clinicians is that the combination of MRI techniques with 3D printing allows for a better understanding of the complexity of patient perianal CD by exact 1 : 1 scale visualisation of fistula anatomy and or abscess together with its relation to the structure of perianal muscles as well as evaluation of both internal and external openings and evaluation of fistula content. All the above-mentioned advantages potentially guide the decision process and enhance both planning and performance of complex surgical procedures. There can also be other advantages. For example, 3D modelling can play an important role in the education of young professional surgeons and gastroenterologists for whom the proper interpretation of DICOM images obtained from traditional MRI imaging might be challenging. This particular method of perianal CD visualisation could be of particular usefulness for educational purposes because it is relatively easy to perform and cost-effective, especially when compared to traditional cadaveric studies.
The process of shared decision-making includes the possibility that the patient will make an informed decision to accept the surgeon’s recommended plan. It is of great importance to ensure that the patient is fully informed and demonstrates a full understanding of a proposed method of treatment. It was clearly demonstrated in laryngology and cardiology that a patient’s understanding of a surgical procedure can be significantly better after showing a 3D model describing the surgical approach before the surgery [25, 26].
Last but not least, promising results of recent and ongoing studies utilising stem cell therapy – either allogeneic or autologous – for treatment of perianal CD opens a new perspective for novel 3D visualisation based on MRI [27]. Although clinical study protocols are not unified in terms of visualisation techniques used, it is obvious that this complex multi-step procedure will require a precise, simple-to-perform, and reproducible radiological technique for technical planning of the procedure as well as a simple tool helping to recruit and obtain patient consent.