Introduction
Endomyocardial biopsy (EMB) of the right ventricle (RV) and left ventricle (LV) was implemented into clinical practice in 1963 by Sekiguchi and Konno [1]. Over the years, this has become a technique of choice for diseases of the myocardium that can be observed at a tissue level and where no other diagnostic techniques can determine the cause of the disease. Moreover, histological and molecular diagnosis allows the administration of a therapy specific for myocardial disease [2]. Nevertheless, an EMB still remains controversial for some physicians due to the invasive nature of the procedure and the risk of complications [3].
The scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology published in 2007 defined the role of an EMB in the management of cardiovascular disease [4]. In the recent position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases published in 2013, the recommendations for an EMB were significantly extended, including for patients with clinically suspected myocarditis, defined as the presence of ≥ 1 clinical presentation (acute pseudoischemic pain, new-onset or signs of worsening heart failure (HF), arrhythmia symptoms, unexplained cardiogenic shock) and ≥ 1 diagnostic criteria (new electrocardiogram, 24-hour Holter monitoring or stress test abnormalities; elevated troponin T/troponin I mass concentration, new abnormalities on cardiac imaging, changes in the muscle tissue in cardiac magnetic resonance (CMR) imaging in the absence of angiographically detectable coronary artery disease (CAD) and known pre-existing cardiovascular disease or extra-cardiac causes that could explain the syndrome [5]. This change broadened the potential number of patients requiring an EMB, especially with selective LV involvement in imaging examinations. However, the proposed criteria are based on the consensus of experts and require a critical approach until they are evaluated in subsequent registers and multicenter randomized trials.
The role of LV-EMB has increased in recent years. In spite of that, the number of EMBs performed in Poland is low. According to the data of the Association of Cardiovascular Interventions of the Polish Cardiac Society, 740 EMBs were performed in Poland in 2014 [6], mostly in patients after orthotopic heart transplantation (OHT).
We observe an increasing number of patients admitted to hospitals due to HF of unknown etiology. Crucial for successful treatment is determining the etiology of the disease, especially in patients with suspected myocarditis or unknown cardiomyopathy. Therefore, since August 2016, in the 3rd Department of Cardiology in Zabrze we have implemented a policy for patients with HF of unknown origin based on the outcomes of LV-EMBs.
Aim
The aim of the study was to present the results of our diagnostic and therapeutic management based on LV-EMB examinations in patients with unexplained HF.
Material and methods
Our study involved patients admitted to the hospital between August 2016 and March 2019 due to HF or cardiomyopathy of unknown etiology in whom an LV-EMB was performed.
All patients underwent a clinical examination, laboratory tests, a resting 12-lead electrocardiogram, chest X-ray, Holter monitoring and 2-dimensional echocardiographic studies. Echocardiographic parameters were calculated and interpreted by an experienced operator according to established criteria. Cardiac magnetic resonance imaging was performed in patients without any contraindications with a 1.5 T scanner using a multi-channel body-array coil as a receiver. T1, T2 maps and late-gadolinium enhancement images were analyzed by an experienced operator, and the CMR findings were consistent with myocardial inflammation if at least two Lake Louise Consensus Criteria were present [7]. EMBs were only performed in patients without significant CAD and without severe valvular disease.
Significant CAD was defined as hemodynamically significant stenosis in the coronary arteries with a diameter ≥ 2.0 mm. A ≥ 50% stenosis of the left main artery or the proximal segment of the left anterior descending artery and a ≥ 70% stenosis in other segments was considered as hemodynamically significant.
All patients were optimally treated, according to the current guidelines for heart failure. Stable patients were on standard pharmacological therapy. Inotropic agents, vasopressors and intra-aortic balloon pumps were given to patients with marked hypotension. The indications for an LV-EMB were unexplained HF with an LV ejection fraction < 35% and a) hemodynamic compromise or electrical instability of the heart; b) a recent worsening of heart failure symptoms (NYHA class II, III or IV) and no response to ordinary care within 2 weeks. Patients with a thrombus in the LV were excluded.
Before an EMB, every patient was treated with 75 mg of aspirin. All EMBs were performed through the femoral artery. Upon the introduction of a 7 Fr (Balton) sheath, every patient received a bolus of unfractionated heparin to achieve an activated clotting time of 200–250 s to prevent thromboembolism during the procedure. A 5 Fr pigtail catheter (Boston Scientific, USA) was then advanced into the left ventricle. After that, a long J-wire (260 cm, 0.03500) was advanced over the pigtail catheter to hold the ventricular position, the pigtail was subsequently removed and an 8 Fr multipurpose guiding catheter (MB 2, 90 cm, Launcher, Medtronic) was carefully advanced over the wire into the left ventricle [8]. The J-wire was removed and a Y connector (Balton) was connected.
The optimal position and the distance between the guiding catheter tip and the lateral LV wall were checked in the left anterior oblique 20° projection by injection of 5–6 ml of a contrast agent [8]. Using a Cordis 104 cm length bioptome (Cordis), 6–8 samples of 1–2 mm were taken during the procedure. To prevent an air embolism, the bioptome forceps were washed in saline before every insertion into the guiding catheter via the Y connector. After completion of the procedure, the 7 Fr sheath was removed and in the majority of patients, a vascular closure device was applied to achieve hemostasis.
Samples for histology and immunohistochemical analysis were fixed in 4% formalin and the bioptates for virus genome analysis were stored in RNAlater (Ambion, Foster City, CA, USA) tubes at room temperature. The material was analyzed at the Department of Molecular Pathology, University Hospital Tübingen, Germany. For histological examinations, 4–5 µm-thick sections of paraffin-embedded biopsies were prepared. Routine diagnostics included hematoxylin and eosin, Masson’s trichrome and Giemsa staining. Histological examination assessed the presence of cardiomyocyte changes, scars, fibrosis, pathological vascular conditions, granulomas and inflammatory cell differentiation. The morphological criteria for myocarditis were based on the detection of inflammatory infiltrates and the presence of myocyte degeneration and necrosis according to Dallas criteria [9].
The histological analysis was supplemented by immunohistochemistry for the evaluation of an ongoing inflammatory reaction. Immunohistochemical diagnostics were based on the application of specific primary antibodies and secondary antibodies were conjugated with an enzyme complex (avidin-biotin-peroxidase complex; Vectastain-Elite, ABC Kit, Vector Laboratories, Burlingame, CA, USA), producing a precipitating colored complex by using a staining solution [10]. Elevated inflammatory cell subsets and increased expression of adhesion molecules were detected using antibodies: CD3 for T-cells (Novocastra Laboratories, Newcastle, UK), CD68 for macrophages (DAKO, Glostrup, Denmark), and HLA-DR-α (DAKO, Hamburg, Germany) to assess major histocompatibility complex class II expression in antigen-presenting immune cells [10]. Previously published criteria were used for the immunohistochemical diagnosis of myocardial inflammation [5, 10–12]. The presence of more than 14 leukocytes/mm2 including ≥ 7 cells/mm2 CD positive T-lymphocytes, CD68 positive macrophages and up-regulation of HLA class II was considered diagnostic for an abnormal inflammatory infiltrate, suggesting myocarditis [5]. The presence of myocyte necrosis with associated inflammatory infiltration based on an immunohistochemical assessment indicated acute myocarditis [12]. In the case of chronic myocarditis (formerly called borderline myocarditis), acute myocyte injury could not be found, but the extent of the inflammation and interstitial fibrosis was confirmed [12].
The molecular diagnostics were based on the detection, quantification and sequencing of viral genomes using methods which rely on polymerase chain reaction (PCR). DNA and RNA were extracted using proteinase-K digestion followed by extraction with phenol/chloroform. Enteroviruses (coxsackieviruses and echoviruses), parvovirus B19, Epstein-Barr virus, human herpesvirus type 6 and 7, cytomegalovirus, influenza virus A and B, varicella-zoster virus and adenoviruses were evaluated by nested PCRs from RNAlater-fixed endomyocardial biopsy samples, and RNA was transcribed into cDNA by reverse transcriptase according to the protocol of the manufacturer (AGS, Heidelberg, Germany) as described [10]. The enzymatic amplification of cDNA or DNA was performed as a nested PCR on a Perkin-Elmer GeneAmp PCR System 9600 (Applied Biosystems, Weiterstadt, Germany) in two 30-cycle programs [10]. Oligonucleotide sequences from the GAPDH gene were used as an indicator of correct isolation of the nucleic acids. Detection of the viral genome by PCR and specificity of all viral amplification products confirmed by automatic DNA sequencing were conditions for recognizing a viral infection [10].
Steroids were administered for patients with active myocarditis, without the presence of a virus, without acute myocyte injury, in the absence of clinical improvement or worsening despite typical HF treatment, with no contraindications for immunosuppression, when the minimum time from the onset of symptoms was 4 weeks or giant cell or eosinophilic myocarditis was found in hemodynamically unstable patients. The prednisone dose was 1 mg/kg daily (a maximum dose of 70 mg). After 4 weeks of treatment, the steroids were tapered each week by 5 mg until the dose was 20 mg/day. Immunosuppressive treatment was recommended for 3 months. Patients were further assessed during subsequent hospitalizations or outpatient visits.
The study was approved by the local institutional Ethics Committee and the patients gave their written informed consent.
Statistical analysis
Descriptive statistics were prepared based on patients’ clinical characteristics, treatment, and the outcome information for registered patients. When normal distribution was observed, the results were presented as the mean ± SD standard deviation. The median and interquartile range are applied in other cases. Categorical data are presented as frequency and proportion (%). The differences between the groups were assessed with the t-test for normally distributed data, while the Mann-Whitney test was used for comparisons between non-normally distributed continuous variables and Fisher’s exact test was used for categorical variables. Cox proportional hazards models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs). A p-value of less than 0.05 was considered as significant. Statistical analyses were performed using the SPSS software package, v. 16.0.
Results
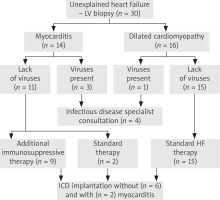
The baseline demographic and clinical characteristics of 30 patients in whom LV-EMB was performed are shown in Table I. The mean ± SD age of patients was 38.9 ±7.6 years and 83.3% of subjects were male. About 75% of patients were in NYHA functional class III or IV on admission, with 8 of them being in cardiogenic shock. Four patients required intra-aortic balloon pump support. The mean ± SD LV ejection fraction was 19.6 ±7.1%. CMR imaging was performed in 26 patients, and active myocarditis was present in 24. There were no procedural complications during either coronarography or the LV-EMB. In 13 patients, coronarography was performed in other hospitals prior to admission to the Silesian Center for Heart Disease; therefore this examination was not repeated. Based on the histological and immunohistochemical criteria, myocarditis was confirmed in 14 patients. Four patients, including 3 with myocarditis, had evidence of a viral infection in their EMB (2 patients with both parvovirus B19 and human herpesvirus 6, 2 patients with only parvovirus B19). In the remaining 16 patients without myocarditis, dilated cardiomyopathy and interstitial fibrosis were found. All patients received standard treatment of heart failure. In 9 patients, steroid therapy was implemented. A cardioverter-defibrillator was implanted in 8 patients. Figure 1 shows the clinical management for patients according to the results of the biopsy. There were no deaths during hospitalization. The mean ± SD period of hospitalization was 30.5 ±10.7 days. During the mean ± SD observational period of 10.5 ±8.1 months all patients survived, 11 patients had an LVEF of over 35%, of which 6 (66%) were on additional immunosuppressive therapy and 5 (24%) on standard HF therapy. An increase of > 20 percentage points in the absolute EF was observed in 7 patients; 4 (44%) were on steroid therapy and 3 (14%) on standard HF therapy. Table II presents the results stratified according to the confirmation of myocarditis and the applied treatment.
Table I
Demographics and clinical characteristics of the study group
[i] Values presented as means ± standard deviation, median (interquartile range) or number (percentage) of patients. AST – aspartate aminotransferase, ALT – alanine aminotransferase, BMI – body mass index, CK-MB – creatine kinase isoenzyme MB, IQR – interquartile range, LA – left atrium, LVEDD – left ventricle end-diastolic diameter, LVEDV – left ventricle end-diastolic volume, LVESD – left ventricle end-systolic diameter, LVESV – left ventricle end-systolic volume, LVEF – left ventricular ejection fraction, NT-proBNP – N-terminal pro-B-type natriuretic peptide, NYHA – New York Heart Association, RVSP – right ventricular systolic pressure, TAPSE – tricuspid annular plane systolic excursion, N/A – not applicable.
Table II
Results stratified according to confirmation of myocarditis and applied treatment
Discussion
An EMB provides a wide diagnostic spectrum of cardiac dysfunction at a cellular level but it is available only in a limited number of centers. Moreover, the number of procedures and the indications for an EMB differ even between experienced centers. In the Silesian Center for Heart Diseases in Zabrze, 483 EMBs were performed in 2017. From 1986 to 2017, 12,423 EMBs were performed in our center. The majority of these were performed in patients after an OHT, and less frequently in patients with myocarditis, cardiomyopathies [13] and structural heart diseases. All of these were RV-EMBs. The diagnostic value of an LV, versus an RV-EMB, has been assessed in various studies [12, 14]. Chimenti et al. compared the diagnostic accuracy of LV-EMBs and RV-EMBs in patients who had a biventricular (BV) EMB. The diagnostic yield of LV-EMBs was superior to RV-EMBs (96.3% vs. 71.4%, p < 0.001) [2]. Escher et al. compared the diagnostic value of LV versus RV-EMB specimens taken simultaneously in patients with suspected myocarditis. They demonstrated that LV-EMBs had a significant diagnostic advantage when interstitial fibrosis, cardiac remodeling, and hypertrophy were investigated. Pronounced or severe fibrosis in specimens from left and right ventricles was determined in 27.6% and 4.6% of patients, respectively. Morphological changes were found to be more reliably determined in LV-EMBs [14]. The presence of fibrosis in patients with dilated cardiomyopathy was associated with 2.43-fold higher odds of all-cause mortality and 3.22-fold higher odds of cardiovascular death or cardiac transplantation [15]. There are several studies reporting the benefits of BV-EMBs [2, 3, 12]. Yilmaz et al. demonstrated the diagnostic advantage of a BV-EMB compared to the selective univentricular procedure [3]. Stiermeier et al. assessed the diagnostic value of implementing a routine BV-EMB approach in 127 consecutive patients with suspected myocarditis. A BV-EMB provides better diagnostic performance compared to a selective RV or LV-EMB. Moreover, a selective LV-EMB was revealed to be superior to a selective RV-EMB for the confirmation or rejection of myocarditis [12].
The main reason for the low number of EMB procedures in the majority of centers is the fear of complications. In most previous studies, a low major complication rate for both LV- and RV-EMBs was reported. Some authors suggested a slightly superior safety profile for LV-EMBs [2, 3, 12, 16]. The overall complication rate varies from 1% up to 6% for centers taking samples mainly from the RV septum, including sometimes large numbers of repetitive EMBs in patients after an OHT [17]. In a report from a single-center study that involved 3,048 RV-EMBs, the risk of major complications including pericardial tamponade with pericardiocentesis, complete atrioventricular block with a permanent pacemaker, urgent cardiac surgery, advanced cardiac life support, hemothorax or pneumothorax was 0.12%, and no deaths were reported [16]. A LV-EMB also seems to be safe. Chimenti et al. found for 3,068 patients who had RV-EMBs and 3,549 patients who had LV-EMBs between 1983 and 2010 that major complications appeared in only 0.45% and 0.36% of patients, respectively. A perforation with cardiac tamponade was noted in 3 (0.08%) patients who had LV-EMBs and in 9 (0.29%) patients who underwent RV-EMBs. There was no death, permanent atrioventricular block or pulmonary embolization [2]. The experience of the physicians and the number of EMBs performed in the center play a crucial role in the low rate of complications. In Stiermeier et al.’s study, previous experience with the LV-EMB approach was the main reason for the low complication rate after implementation of the BV-EMB procedure [12]. Our 30 years of expertise in RV-EMBs contributed to avoiding major complications in the course of carrying out LV-EMBs, although the number of patients was limited.
Diagnosing myocarditis and determining the etiology of HF are often extremely challenging. Clinical symptoms, results of laboratory tests, electrocardiogram and echocardiography are often insufficient for establishing a proper diagnosis. Although CMR seemed to be a promising non-invasive approach, “real life” revealed the limitations of this method [18]. An endomyocardial biopsy is particularly worth considering in patients during the first weeks after the onset of the disease, especially in the cases of young people, and with those with severely worsening symptoms of HF. Based on the current clinical trials, there is no unambiguously established way to proceed in relation to the treatment directed to the causative agent, and in each situation, the risks and benefits of such treatment should be individually considered. The above work presents the possibilities and effects of using this type of treatment in our center. Analysis of the bioptates enables us to provide information about the presence of viral genomes, to differentiate between acute and chronic myocarditis and, consequently, to have an impact on therapeutic decisions [5, 19, 20]. Frustaci et al. demonstrated in 85 patients with myocarditis and chronic HF that inflammation recognized by immunohistology and the absence of viral genomes confirmed by polymerase chain reaction allowed immunosuppression to be used, with a significant 6-month improvement in LV function [20]. This confirms the benefits of EMB as a diagnostic tool. Therefore, we commenced our program in close cooperation with the diagnostic center in Tübingen using their experience in this area. We obtained an immunohistological evaluation which was not only descriptive but also quantitative (number of cells/mm2); therefore the results were unambiguous in terms of diagnosing inflammation and they also contained specific recommendations for further treatment (immunosuppression, antiviral, optimal HF therapy). In our study, 66% of patients with additional immunosuppressive therapy and 24% with standard HF therapy had an improvement in LVEF of over 35% (HR = 2.85; p = 0.08). Currently, immunosuppression may be considered, on an individual basis, for infection-negative lymphocytic myocarditis, with no response to standard HF therapy, in patients with no contraindications to immunosuppression [5].
The main findings of the present study can be summarized as follows. Firstly, we demonstrated the presence of myocarditis in about 46% of patients with unexplained HF with reduced EF. This is a result comparable to previous data. Mavrogeni et al. reported that 48% of patients with the diagnostic criteria for clinically suspected myocarditis had positive immunohistological criteria in an EMB [21]. Secondly, only 13.3% of our patients had the presence of the viral genome confirmed. A similar result was observed in another study in patients with dilated cardiomyopathy [22]. In contrast, Kühl et al. revealed the detection of viral genomes in 67% of patients clinically presenting with “idiopathic” dilated cardiomyopathy (DCM) [23]. Finally, the exclusion of viral persistence enables the implementation of immunosuppressive therapy, and this is one of the main advantages of biopsy over cardiac magnetic resonance [5]. In this way, a left ventricular endomyocardial biopsy changed the therapeutic management in 30% of our patients. EMBs should be considered in the diagnosis of patients with suspected myocarditis as the only method that identifies the etiology, the type of inflammatory infiltrates and the duration of inflammation which is associated with different prognoses and treatments [5, 20]. Confirmation of the active inflammatory process in the myocardium is one of the factors that should be taken into account when deciding the most appropriate time to implant a cardioverter-defibrillator. The correct diagnosis allows the implementation of adequate, targeted treatment, which is often the only chance to avoid severe chronic heart failure.
The major limitation is the low number of enrolled patients. Patients involved in the analysis were relatively young with a low number of comorbidities and may not correspond to the entire population of patients with HF of unknown etiology.
Conclusions
An LV-EMB performed by skilled physicians in an experienced center is a safe procedure. If the diagnostic tools are provided, it may be crucial for determining the origin of HF. An LV-EMB verifies the cardiac magnetic resonance results, and, as the diagnostic gold standard, it reduces the total diagnostic time and allows the use of additional therapeutic options in a large group of patients with severe impairment of LV systolic function.