Introduction
Breast cancer (BC) is not only the most frequently diagnosed malignant tumour in females worldwide (representing 30% of all new cancer cases) but also the second cause of death from cancer (15% of all cancer mortality in women) [1]. Epidemiological data indicate that in Poland in 2020, BC was the most prevalent malignant tumour with a morbidity rate of 23.8% [2]. Each year, more than 22,000 women are diagnosed with breast cancer, with 5–10% being diagnosed at an advanced stage. The combination of self-examination and early screening practice has led to an increase in the number of patients diagnosed at an early stage of the disease [3]. Study results suggest that the use of adjuvant chemotherapy (CT) may lead to a decrease in the risk of relapse and mortality in early BC [4]. However, CT appears not to be equally beneficial in all patients [5]. Hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) breast cancer, which occurs in approximately 70% of all breast cancers, shows an over 94% 5-year overall survival rate, partly because of a low tumour recurrence rate after resection and adjuvant CT [6–10]. Nevertheless, data show that in HR+, HER2– breast cancer, up to 85% of patients may only marginally benefit from CT while being exposed to its toxicities [10, 11]. Therefore, the optimal treatment of early HR+, HER2–, and lymph node-negative (N0) BC remains controversial [12]. As BC is a heterogeneous disease, some patients are sufficiently treated by endocrine therapy (ET) alone, while others require CT to reduce recurrence rates and improve survival [13]. Therefore, the individual estimation of distant recurrence risk and the expected benefits from adjuvant CT should be considered when deciding on adequate treatment [14, 15]. Stratifying patients based on their individual recurrence risk allows for a personalised and optimised treatment approach [16, 17]. The risk of recurrence is estimated using clinicopathological factors such as age, tumour size and grade, lymph node status, and HR and HER2 status. However, clinical practice indicates that clinicopathological features alone are only a prognostic tool and appear to be inadequate to determine the CT benefit, thus potentially leading to overtreatment or undertreatment of a large percentage of patients [16, 18]. The effectiveness of CT has primarily been shown to be independent of clinicopathological factors [9]. Due to the absence of reliable predictive indicators, individuals with high-clinical-risk BC might receive excessive treatment, potentially leading to unnecessary side effects. Conversely, patients with low-clinical-risk BC typically do not undergo routine CT, which could put certain patients at risk of receiving inadequate treatment [18, 19]. In response to this need, gene expression profiling tests have been developed, including the 50-gene Prosigna® Breast Cancer Assay, 12-gene EndoPredict® assay, 7-gene Breast Cancer Index®, 70-gene MammaPrint® assay, as well as the 21-gene Oncotype DX Breast Recurrence Score® assay [20–22].
The Oncotype DX Breast Recurrence Score® test (Exact Sciences Corporation, Madison, WI, USA) is a multigene assay based on the expression of 21 genes of predictive and prognostic value (16 cancer-related genes: Ki-67, STK15, Survivin, Cyclin B1, MYBL2, GRB7, HER2, ER, PGR, BCL2, SCUBE2, Stromelysin 3, Cathepsin L2, GSTM1, CD68 and BAG1) and 5 reference genes (ACTB, GAPDH, GUS, RPLPO, and TFRC) [5]. It has been validated in large prospective randomised clinical trials, including TAILORx (Trial Assigning Individualized Options for Treatment) and RxPONDER (A Clinical Trial RX for Positive Node, Endocrine Responsive Breast Cancer) to anticipate the risk of BC recurrence (prognostic value of the test) as well as predict the benefit from adjuvant CT (predictive value of the test) [18, 23–25]. The prognostic component enables the evaluation of the recurrence risk at 9 years for N0 and 5 years for N1, while the predictive component assesses the anticipated benefit of adjuvant CT. This test is intended for use in patients with HR+, HER2– early BC with up to 3 positive lymph nodes (N0 and N1) [4].
Because there is a lack of data on the impact of the Oncotype DX Breast Recurrence Score® test on adjuvant CT decisions in Poland, the purpose of the PONDx survey was to assess the real-life clinical utility and decision impact of the Oncotype DX Breast Recurrence Score® result in patients with HR+, HER2– early BC with N0 disease, even though this test is currently not being reimbursed in Poland. However, it is reimbursed in several countries worldwide, including Germany, France, the Netherlands, Switzerland, Italy, and Ireland and has led to a significant reduction in CT administration and financial savings in public healthcare systems [26–29].
Material and methods
Study group
The PONDx survey investigated the real-life use of the Oncotype DX Breast Recurrence Score® test and was conducted in 8 clinical reference centres in Poland February 2021 – July 2022. The trial was set up to further characterise the Polish HR+, HER2–, N0 early BC population and evaluate the impact of the Oncotype DX Breast Recurrence Score® results on physicians’ decisions concerning adjuvant CT. Eight clinical reference centres in 7 Polish cities (Białystok, Gdańsk, Gliwice, Kraków, Poznań, Szczecin, Warsaw) participated in this survey. Patients with HR+, HER2–, node-positive cancer, as well as those with HR– and/or HER2+ breast cancer, were excluded from the study.
Physicians participating in this trial used an electronic case report form to document patient characteristics (age and menopausal status), tumour characteristics (histological subtype, tumour size, grade – G), lymph node status (sentinel lymph biopsy), Oncotype DX Breast Recurrence Score® result, and changes in treatment from CT followed by ET (CT + ET) to ET alone and from ET alone to CT + ET based on the Oncotype DX Breast Recurrence Score® test result.
Statistical analysis
Descriptive statistics were used to summarise patient and tumour characteristics. The relative change in CT + ET was calculated as the difference in the proportion of patients with CT + ET treatment decisions post-assay vs. pre-assay. The absolute change in CT + ET was calculated as the difference in the proportion of patients with CT + ET treatment decisions post-assay vs. pre-assay divided by the pre-assay proportion. McNemar’s test was used to compare the proportion of patients who received CT post-assay vs. those recommended CT pre-assay (overall, by Oncotype DX Breast Recurrence Score® result, menopausal status, and tumour histology). χ2 tests or Fisher’s exact tests were used to compare changes in treatment according to patient and tumour characteristics.
Results
Patient and tumour characteristics
The survey included 204 patients with HR+, HER2–, N0 breast cancer. Patient and tumour characteristics overall and separately for patients with a pre-assay CT + ET recommendation, patients with an Oncotype DX Breast Recurrence Score® result greater than 25, and patients with a change in treatment from pre- to post-assay are summarised in Table 1.
Table 1
Patient and tumour characteristics overall and in the subgroups with a pre-assay recommendation for CT + ET, with a high Oncotype DX Breast Recurrence Score® result, and with treatment change from pre- to post- Oncotype DX Breast Recurrence Score® result
More than half of the surveyed patients were 50 years of age or older (n = 117, 57.4%); however, a substantial number of younger patients were also included (< 40 years 13.7%, 40–49 years 28.9%). A total of 52.9% of patients were post-menopausal and 42.2% were pre-menopausal. Ductal carcinoma was the most common histology (n = 161; 78.9%), followed by lobular cancer (n = 35; 17.2%). Mixed, mucinous, and other cancer types were observed in 2%, 0.5%, and 1.5% of patients, respectively. Most patients had G2 tumour (n = 133, 65.2%), 41 patients (20.1%) had G1, and 30 patients (14.7%) had G3 tumour. The Ki-67 expression was greater than 20% in 120 patients (58.8%). Most patients had a low Oncotype DX Breast Recurrence Score® result of 0 to 25 (n = 144, 70.6%), while 58 patients (28.4%) had a high Oncotype DX Breast Recurrence Score® result of 26 to 100. There were 2 patients with missing Oncotype DX Breast Recurrence Score® results. Of patients with an Oncotype DX Breast Recurrence Score® result greater than 25, 31.0% were aged 60–69 years (n = 18), 25.9% were aged 40–49 years (n = 15), and 24.1% were aged 50–59 years (n = 14). Regarding the histology, 86.2% of patients with a high Oncotype DX Breast Recurrence Score® result had ductal BC and 13.8% had lobular breast cancer. Furthermore, 65.5% of patients with a high Oncotype DX Breast Recurrence Score® result had G2 cancer, while 25.9% and 8.6% had grade G3 and G1 cancer, respectively.
Decision impact results
Three patients were excluded from the analysis due to incomplete information or administration of treatment other than CT + ET or ET. Changes in treatment decision from pre- to post-assay overall and by Oncotype DX Breast Recurrence Score® result, menopausal status, and histological subtype are described in Table 2.
Table 2
Treatment decisions overall and by Oncotype DX Breast Recurrence Score®, menopausal status, and histological subtype of cancer
In the evaluable population (n = 201), the physicians initially recommended CT + ET in 90 patients (44.8%) before the availability of the Oncotype DX Breast Recurrence Score® result, while 111 patients (55.2%) were assigned to ET alone (Fig. 1).
Fig. 1
Overall reduction in chemotherapy administered based on the Oncotype DX Breast Recurrence Score® result
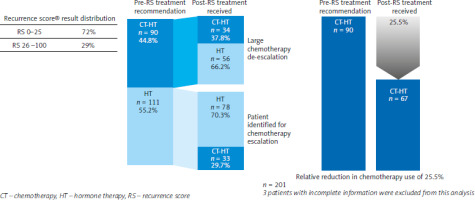
The Oncotype DX Breast Recurrence Score® result had a significant impact on the treatment decision and led to a change of therapy in 44.3% of patients (n = 89, p = 0.015). With the Oncotype DX Breast Recurrence Score® results, the number of patients who received adjuvant CT dropped to 90–67, corresponding to a relative reduction of 25.5% (95% CI: 11.7–2.3) and absolute reduction of 11.4% (95% CI: 1.9–21.0) (Fig. 1). The Oncotype DX Breast Recurrence Score® result led to a treatment de-escalation in 62.2% of patients, who otherwise would have been overtreated, and an escalation of treatment in 29.7%, who otherwise would have been undertreated. Looking at the different Oncotype DX Breast Recurrence Score® groups, there was a significant proportion of patients in whom treatment was changed based on the Oncotype DX Breast Recurrence Score® result. Among patients with an Oncotype DX Breast Recurrence Score® result of 0–25, treatment was de-escalated in 54 patients (37.8%) (p < 0.001) (Table 2). Among patients with an Oncotype DX Breast Recurrence Score® result of 26 to 100, treatment was escalated in 25 patients (43.9%) (p < 0.001).
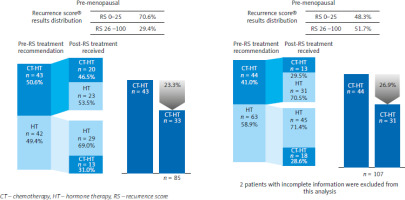
In the evaluable population, the use of Oncotype DX Breast Recurrence Score® resulted in a relative reduction by 29.6% (95% CI: 9.9–73.9) in CT administered in post-menopausal patients and a relative reduction by 23.3% (95% CI: 6.0–67.5) in CT administered pre-menopausal patients (Fig. 2). In the overall population, oncologists changed their treatment decision from CT + ET to ET alone in 29.0% of post-menopausal patients and 27.1% of pre-menopausal patients, although these changes did not reach statistical significance. Of the patients in whom treatment was changed (n = 87), 43.5% were post-menopausal and 40.7% were pre-menopausal, although the difference was not significant (Table 1).
Fig. 2
Reduction in chemotherapy administered based on the Oncotype DX Breast Recurrence Score® test in pre- and post-menopausal patients
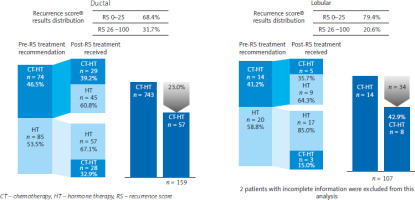
In patients with ductal tumours, treatment was de-escalated from CT + ET to ET in a significant percentage of patients (28.3%; p = 0.047). On the other hand, therapy was de-escalated in 26.5% of patients with lobular tumours, but this result was not significant (Table 2). The rate of overall treatment changes was similar across histological subtypes: 45.9% of patients with ductal tumours and 35.3% of patients with lobular tumours. In 14 patients with lobular tumours, CT was initially recommended, but only 8 of them received CT post-assay, representing a relative reduction by 42.9% (95% CI: 11.1–108). In patients with ductal tumours, the use of Oncotype DX Breast Recurrence Score® resulted in a relative reduction in the CT rate by 23.0% (95% CI: 8.3–55.9) (Fig. 3).
Fig. 3
Reduction in chemotherapy administered based on the Oncotype DX Breast Recurrence Score® test in patients with ductal and lobular breast cancer
While there is no evidence for differences in treatment change across patient or tumour characteristics, the highest proportion of treatment change in the overall population was noted in patients aged 60–69 years (50.0%), patients with G2 tumours (45.9%), and patients with Ki-67 expression greater than 20% (45.0%).
Discussion
The decision regarding adjuvant CT treatment in early BC is usually based on clinicopathological factors, including disease stage, ER and PR expression levels, HER2 status, tumour grading, and Ki-67 expression [30]. However, the development of novel genomic testing tools has led to a reduction in CT burden in patients with HR+, HER2– early breast cancer.
This real-world evidence survey was performed to assess the impact of the Oncotype DX Breast Recurrence Score® result on physicians’ decisions regarding treatment of patients with HR+, HER2– early BC in Poland. As expected, ductal carcinoma was the most common type in our study, followed by lobular cancer. Over half of participants showed high Ki-67 expression levels (≥ 20%). According to the literature, high Ki-67 expression is associated with poor long-term survival [31–33]. In contrast, the utility of Ki-67 expression for predicting the benefit of adjuvant CT is questionable due to the arbitrary nature of Ki-67 assessment and unclear cut-off values for defining high vs. low expression. In 28.4% of participants, the Oncotype DX Breast Recurrence Score® result was ≥ 26, with the highest representation among patients aged 60–69 years.
The Oncotype DX Breast Recurrence Score® result was suggested to aid treatment decision-making in high- or low-risk patients to avoid overtreatment or undertreatment with CT, respectively. In our study, Oncotype DX Breast Recurrence Score® results led to a relative reduction of CT rates in 25.5% of patients. In 62.2% of patients who were initially recommended for a CT-containing regimen, treatment was de-escalated based on their genomic result, while in 29.7% of patients who were identified as genomic high-risk despite being of clinical low risk (based on tumour size and type, etc.), treatment was subsequently escalated to CT. The observed relative reduction was especially pronounced in post-menopausal patients and in those with lobular breast cancer. The utility of Oncotype DX Breast Recurrence Score® test appeared to be somewhat lower in lobular cancers (non-significant decision impact p = 0.08) compared with ductal cancers (p = 0.047). This observation aligns with other studies demonstrating significantly different distribution of Oncotype DX Breast Recurrence Score® results in invasive lobular cancers compared with invasive ductal carcinoma, suggesting that lobular cancers may have unique recurrence patterns and treatment responses [34]. Moreover, the highest proportion of treatment change in the overall population was in patients aged 60–69 years, those with G2 tumours, and with high Ki-67 expression.
The results of our study are in line with previous studies indicating the usefulness of the Oncotype DX Breast Recurrence Score® result with regards to treatment decisions. Bae et al. [35] demonstrated that the application of the Oncotype DX Breast Recurrence Score® result in patients from the Republic of Korea decreased the use of CT by approximately 30–40% in the high-clinical-risk group without affecting survival outcomes. Similarly, a prospective observational study carried out in 27 BC reference centres in 6 regions of Italy demonstrated that Oncotype DX Breast Recurrence Score®-guided treatment decisions resulted in an overall reduction of CT + ET recommendations by 36% in patients with mostly invasive ductal HR+ carcinomas, with histological grade 2 and 3 [36]. Also, the PONDx observational study, which examined the use of the Oncotype DX Breast Recurrence Score® test in routine clinical practice in France, revealed that the availability of Oncotype DX Breast Recurrence Score® result led to a net absolute reduction in CT recommendations by 36% across the entire population and by 29% in patients with G3 tumour and/or Ki-67 expression > 20% [37]. Additionally, it showed that 95% of patients with an Oncotype DX Breast Recurrence Score® result < 18 were recommended ET only, while 97.5% of those with an Oncotype DX Breast Recurrence Score® > 30 were advised to undergo additional CT. On the other hand, a recent Swiss decision impact study revealed that there was an increase of tumour board recommendations for CT in the high Oncotype DX Breast Recurrence Score® category, which resulted in only minor overall changes in the administration of CT. However, in the intermediate Oncotype DX Breast Recurrence Score® category, particularly an Oncotype DX Breast Recurrence Score® of 21–25, a significant reduction from 35–17% was noted [38].
Our study also confirms the results of the prospective randomised clinical study TAILORx in patients with HR+, HER2 – early with N0 disease. In this study, 43% of patients with Oncotype DX Breast Recurrence Score® results of 26–100 had low clinical risk and would have been undertreated if decisions had been solely based on clinicopathological features [18, 23, 24, 39, 40]. These results suggest that the magnitude of CT benefit depends on the Oncotype DX Breast Recurrence Score® result, and it increases with an increasing Oncotype DX Breast Recurrence Score® result [41, 42]. Furthermore, in a retrospective observational study of the National Cancer Database, low Oncotype DX Breast Recurrence Score® results had a negative predictive value of 92.2% for no adjuvant CT, and positive predictive values of 40.1% and 81.2% for adjuvant CT in intermediate and high Oncotype DX Breast Recurrence Score® groups, respectively [43].
Similarly, the RxPONDER trial in a population of patients with ER+ HER2– BC with 1–3 positive lymph nodes reported that in post-menopausal patients with a low Oncotype DX Breast Recurrence Score® result CT could be safely omitted [25]. Next, the multi-centre prospective Canadian study, conducted in a similar patient population, revealed that the Oncotype DX Breast Recurrence Score® result led to a change in treatment decisions in 52% of cases (CT + ET to ET or vice versa), with a net reduction in overall CT use of 45% [44]. In subsequent studies, Hassan et al. [45] reported an overall reduction of CT recommendations by 67%, including a 44% reduction in pre-menopausal Canadian patients and 56% in patients with 2 or 3 positive lymph nodes, especially in low- or intermediate-risk Oncotype DX Breast Recurrence Score® groups.
Our observations shed further light on the intricate interplay between CT efficacy, Oncotype DX Breast Recurrence Score®, and age demographics, underscoring the need for personalised treatment approaches tailored to individual patient profiles. The assessment of Oncotype DX Breast Recurrence Score® has been demonstrated not only to improve treatment decisions and management, but also to enhance physicians’ confidence concerning their treatment recommendations [46–48]. Moreover, the use of the Oncotype DX Breast Recurrence Score® assay has proven to be cost-effective in many countries around the world due to reduced CT use as well as enhanced clinical outcomes [5]. A German study emphasised that the implementation of the Oncotype DX test has reduced healthcare costs by decreasing the use of unnecessary CT and enhanced the cost-effectiveness of progressive disease management [49]. Similar effects can be expected in the Polish population, which would allow a more rational use of financial resources.
Although this study provides important information regarding the Oncotype DX Breast Recurrence Score® testing in Poland, some limitations should be noted. Patients in this study were not randomly selected, which can possibly limit the generalisability of the results. In addition, follow-up outcome data are not available for these patients. Finally, because this study included only N0 patients, further studies are required to determine decision impact in node-positive patients.
Conclusions
The introduction of Oncotype DX Breast Recurrence Score® results significantly influenced treatment decisions, leading to a 25.5% relative and 11.4% absolute reduction in CT rates. Treatment was de-escalated in 62.2% of cases initially recommended for CT and escalated in 29.7% of cases initially recommended for ET only, with the most notable changes seen in post-menopausal women and those with lobular breast cancer. Overall, Oncotype DX Breast Recurrence Score® results improved patient management and reduced the risk of overtreatment or undertreatment, enhancing physicians’ confidence in their recommendations.








