Introduction
In recent years, there has been a significant increase in the prevalence of overweight/obesity among children and adolescents, which has become a global problem. Obesity is a serious metabolic disorder affecting many organs, one of which is the liver. Liver disease associated with metabolic dysregulation was previously defined as non-alcoholic fatty liver disease (NAFLD) [1, 2]. Nowadays, to emphasize the co-occurrence of metabolic syndrome and hepatic steatosis, the name NAFLD has been replaced with metabolic-associated fatty liver disease (MAFLD). It should be noted that steatosis can be associated with the absence of other specific histologic changes, as well as with steatohepatitis, with or without fibrosis [2]. Although it seemed that simple steatosis was mainly observed in the pediatric population, an increasing number of cases of fibrosis and even cirrhosis have been described in children and adolescents with MAFLD [3].
Given the broad spectrum of MAFLD, it is important to select patients with advanced liver disease, such as fibrosis, which is associated with increased morbidity and mortality [4]. Currently, the reference standard for evaluating liver fibrosis is to perform an invasive procedure, which is liver biopsy. Percutaneous liver biopsy carries the risk of complications, pain and sample error associated with evaluating only a small portion of the organ [3]. Given the limitations of widespread use of this method, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommends liver biopsy in specific cases of NAFLD/MAFLD (e.g., to rule out other diseases, in very young children, with suspected advanced disease and before therapeutic intervention) [5]. Consequently, new, low-cost and non-invasive methods of assessing liver fibrosis are being sought, which could be used not only to diagnose liver fibrosis, but also to monitor the progression of the disease. In recent years, there has been an intense search for new methods to assess liver fibrosis. These include simple laboratory tests (for example, aspartate aminotransferase [AST]/platelet ratio [APRI] or fibrosis index-4 [FIB-4]) or imaging techniques (such as transient elastography [TE]) [5]. In children, data on non-invasive biomarkers of liver fibrosis are limited.
Increasing evidence suggests that new potential serum biomarkers may be useful in evaluating liver fibrosis. In a study on adults with NAFLD, levels of growth differentiation factor-15 (GDF-15) were a predictor of liver fibrosis [6]. Another substance of interest to researchers is thrombospondin-2 (TSP2), higher serum levels of which were observed in adult patients with NASH compared to simple steatosis [7]. Similarly, higher serum values of pentraxin 3 (PTX3) were associated with the risk of liver fibrosis in adult patients with NAFLD [8]. Data on levels of angiopoietin-like protein 8 (ANGPTL8), also known as betatrophin, in patients with NAFLD are conflicting [9, 10]. However, Cengiz et al. hypothesized that ANGPTL8 may be a biomarker of significant liver fibrosis in NAFLD [10]. To the best of our knowledge, the aforementioned markers in pediatric patients with MAFLD have not been studied. It is also worth noting that children, compared to adults, are less likely to suffer from comorbidities and use medications. Therefore, the purpose of our study was to evaluate serum levels of GDF-15, TSP2, PTX3 and ANGPTL-8 in children/adolescents with MAFLD as biomarkers of liver fibrosis and hepatocyte damage.
Material and methods
Fifty-six overweight/obese children and adolescents with suspected liver disease (hepatomegaly on physical examination or abnormal liver enzymes or features of hepatic steatosis on abdominal ultrasound) admitted to our department were included in the prospective study. Patients in the study group were then divided into two subgroups: those with MAFLD and patients without MAFLD (non-MAFLD) according to the latest consensus [11]. The control group included 23 children without somatic organ pathology and with normal BMI. The study was approved by the Bioethics Committee of the Medical University of Bialystok (study consent number: APK.002.70.2023). All aspects of this study were conducted in accordance with ethical principles for medical research involving human subjects, in accordance with the Declaration of Helsinki.
Participants were screened for other causes of liver damage. Patients with confirmed hepatitis B, C, cytomegalovirus, Epstein-Barr virus infections and celiac disease (according to appropriate serological tests) were excluded. Selected liver diseases such as autoimmune liver disease, Wilson’s disease, cystic fibrosis and α1-antitrypsin deficiency were ruled out using standard clinical and laboratory criteria. None of the children were taking medications that affected lipid or carbohydrate metabolism. Each patient enrolled in the study underwent a physical examination with anthropometric measurements, including weight and height, from which body mass index (BMI) was calculated and presented as z-scores and percentiles. BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). Children were overweight or obese if their BMIs were ≥ 85th percentile. A bioimpedance analysis (BIA) was conducted to assess body composition using a body fat analyzer (Tanita, Tokyo, Japan). The following measurements were taken: body fat percentage (fat %), fat mass, fat-free mass (FFM), muscle mass and total body water mass (TBW) and percentage (TBW%). In order to obtain the above measurements, data such as gender, age and height had to be entered into the analyzer and then, using formulas, the parameters were automatically calculated.
Hepatic steatosis was diagnosed in abdominal ultrasound. Non-invasive ultrasound TE (FibroScan 502 Touch) was performed by a certified hepatologist to assess liver fibrosis. A minimum of four hours of fasting prior to the testing was required. Measurements were performed on patients lying supine with elevation of the right hand to achieve right rib cage spread. After gel application, the probe was positioned perpendicular to the skin surface in one intercostal space adjacent to the right liver lobe, on the midaxillary line. After adequate positioning, a low frequency shear wave was induced by a probe (M or XL probe according to device indications). On the basis of the velocity and intensity attenuation of the shear wave, the acquired data were processed and displayed on the screen as the liver stiffness measurement (LSM). Unsuccessful measurements were automatically excluded by the device. The final result was based on ten effective measurements. Based on the latest publication, the TE cut-off value for detecting clinically significant fibrosis (> F2 according to the METAVIR fibrosis staging scale) was 7.4 kPa [12].
A blood sample was taken from all patients in our study upon admission to the hospital after a 10-hour fast. All sera were immediately frozen and stored at –80°C until use. Routine biochemical measurements, including total blood levels, alanine transaminase (ALT), AST, γ-glutamyltransferase (GGT), total bilirubin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), uric acid, glucose and insulin, were performed using standard methods. The homeostasis model score [HOMA-IR = (fasting insulin × fasting glucose/22.5)] was calculated to estimate insulin resistance. Non-invasive liver fibrosis scores were also calculated using the following formulas: (AST/AST upper limit of normal)/platelet count [109/l] * 100 for APRI and (age * AST/[platelets (109/l) * √ALT] for FIB-4 [13].
Serum concentrations of biomarkers (GDF-15, TSP2, PTX3 and ANGPTL8) were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s recommendations (ELISA Kits, Cloud-Clone Corp., Katy, USA).
Statistical analysis was performed using IBM SPSS Statistics 27.0 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). Continuous variables were presented as medians and 1st-3rd quartiles (Q1-Q3). Frequencies and percentages were given for categorical variables. For comparisons we used the Mann-Whitney test and χ2 test for continuous and categorical variables, respectively. Spearman correlation was performed to measure the correlations between variables. Receiver operating characteristics (ROC) analysis was performed to calculate the power of selected parameters to detect MAFLD in obese children. Areas under the curve (AUCs) were compared using statistical tests against a value of 0.5, representing the diagonal line of no information on the ROC plot. The optimal cut-off values were determined by maximizing the Youden index. In multivariable logistic regression odds ratios (ORs) with the corresponding 95% confidence interval (95% CI) were calculated based on those variables identified as statistically significant by group comparison tests and ROC analysis. Statistical significance was defined as p < 0.05.
Results
Patients in the study group showed significantly different concentrations of GDF-15 (p = 0.002) and PTX3 (p = 0.001) compared to the control group (data not shown). Among 56 obese children, 31 (53.36%) with liver steatosis in ultrasound examination were diagnosed with MAFLD. Table 1 shows the characteristics of the patients stratified by MAFLD presence. ALT, AST, GGT, HDL cholesterol, APRI and LSM values were significantly higher in children with MAFLD compared to the group without MAFLD. Moreover, patients with MAFLD showed significantly elevated levels of TSP2 compared to children without MAFLD (Table 1).
Table 1
Characteristics of obese patients stratified by metabolic-associated fatty liver disease (MAFLD) status
[i] ALT – alanine aminotransferase, AST – aspartate aminotransferase, GGT – γ-glutamyltransferase, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, TG – triglycerides, HOMA-IR – homeostasis model assessment score, APRI – AST/platelet ratio index, FIB-4 – fibrosis-4, BMI – body mass index, fat % – body fat percentage, FFM – fat mass, fat-free mass, muscle mass, TBW% – total body water mass (TBW) and percentage, LSM – liver stiffness measurement, GDF-15 – growth differentiation factor-15, TSP2 – thrombospondin-2, PTX3 – pentraxin 3, ANGPTL8 – angiopoietin-like protein 8, WHR – waist to hip ratio
In the study group, there was a significant positive correlation between TSP2 and ALT (r = 0.48, p < 0.001), AST (r = 0.36, p = 0.007), GGT (r = 0.44, p = 0.001), TG (r = 0.31, p = 0.02), insulin (r = 0.28, p = 0.04) and APRI values (r = 0.49, p < 0.001). Other positive correlations were: GDF-15 and ALT (r = 0.34, p = 0.01), AST (r = 0.27, p = 0.04), uric acid (r = 0.34, p = 0.01); a negative correlation was ANGPTL8 and HDL-cholesterol (r = –0.33, p = 0.01). Nevertheless, LSM did not correlate with GDF-15, TSP2, PTX3 and ANGPTL8.
The ROC analysis presented in Table 2 was performed to determine which potential markers of fibrosis had the best predictive value for distinguishing overweight/obese children with MAFLD from those without MAFLD. The results with the highest statistical significance were obtained for APRI (p = 0.003, AUC = 0.7174 with 87.1% sensitivity and 60.0% specificity at the cut-off level 0.21) and LSM (p = 0.03, AUC = 0.6832 with 80.8% sensitivity and 47.4% specificity at the cut-off level 5.2). The AUC of serum TSP2 (cut-off = 4.3 ng/ml, sensitivity = 53.3%, specificity = 80.0%) to predict MAFLD in obese children was 0.6827 and was statistically significant at p = 0.01 (Table 2). GDF-15, PTX3 and ANGPTL8 were not useful.
Table 2
Analysis of the diagnostic efficiency of selected parameters
When potential parameters were examined to determine which ones might be associated with an increased likelihood of MAFLD diagnosis, LSM was the only significant factor found in a multivariate logistic regression (OR = 2.043, 95% CI: 1.033-4.044, p = 0.04) (Table 3). The next step was to create a multivariate regression model incorporating factors that were significant in the ROC analysis (APRI, LSM, TSP2). Again, LSM was the only significant parameter associated with the diagnosis of MAFLD in children (OR = 1.862, 95% CI: 1.033-3.357, p = 0.04) (Table 4, Fig. 1).
Fig. 1
Receiver operating characteristics (ROC) curve of the ability of markers to differentiate patients with metabolic-associated fatty liver disease (MAFLD) from patients without MAFLD. Aspartate aminotransferase (AST)/platelet ratio index (APRI), fibrosis-4 (FIB-4), liver stiffness measurement (LSM), growth differentiation factor-15 (GDF-15), thrombospondin-2 (TSP2), pentraxin 3 (PTX3) and angiopoietin-like protein 8 (ANGPTL8)
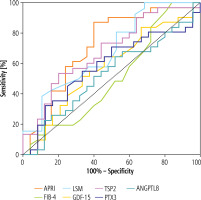
Table 3
Potential parameters of fibrosis in children with MAFLD analyzed using a multivariate logistic regression model
| Parameters | p | OR | 95% CI |
|---|---|---|---|
| APRI* | 0.23 | 1.665 | 0.727-3.814 |
| FIB-4* | 0.56 | 0.705 | 0.216-2.300 |
| LSM | 0.04 | 2.043 | 1.033-4.044 |
| GDF-15 | 0.57 | 0.991 | 0.961-1.022 |
| TSP2 | 0.50 | 1.190 | 0.715-1.980 |
| PTX3 | 0.50 | 1.001 | 0.998-1.004 |
| ANGPTL8 | 0.61 | 0.790 | 0.314-1.987 |
Table 4
Parameters of fibrosis in children with MAFLD de-selected by ROC analysis analyzed using a multivariate logistic model
| Parameters | p | OR | 95% CI |
|---|---|---|---|
| APRI* | 0.18 | 1.545 | 0.815-2.932 |
| LSM | 0.04 | 1.862 | 1.033-3.357 |
| TSP2 | 0.39 | 1.222 | 0.777-1.920 |
Discussion
Liver fibrosis is associated with excessive deposition of extracellular matrix proteins, which leads to scarring of tissues, disruption of the architecture of organ, resulting in its dysfunction. Regardless of the cause, fibrosis/cirrhosis is the end stage of chronic liver disease representing a significant problem worldwide [14, 15]. It is important to assess the presence and severity of liver fibrosis as early as possible in all patients with MAFLD. The gold standard for evaluating liver fibrosis is liver biopsy with direct visualization of fibrosis. As mentioned in the introduction, this procedure has limitations that include sampling error due to small sample size, inter-observer variability, complications and cost. In addition, in children, liver biopsy is associated with general anesthesia. Minimally invasive, safe, reproducible methods are being sought to detect hepatic fibrosis, as well as to monitor the process. Therefore, TE is an increasingly valuable method in the assessment of liver fibrosis.
This study found significantly higher LSM, APRI and TSP2 values in obese children with MAFLD compared to the non-MAFLD group. Similarly, in ROC analysis, the best differential values between the MAFLD group and the group without MAFLD were obtained for LSM, APRI and TSP2, with the highest area under the curve value for APRI. However, logistic regression models including the investigated markers showed that only an increase in LSM significantly raised the risk of being diagnosed with MAFLD in obese children. Similarly to us, Yang et al. observed higher values of LSM in children with NAFLD compared to simply obese contemporaries. In addition, this study positively evaluated the usefulness of TE in the non-invasive diagnosis of NASH in children [16]. Other studies have also demonstrated the utility of TE in the assessment of liver fibrosis in NAFLD/MAFLD patients, but it is worth noting that the indicated cut-off points of significant and advanced fibrosis varied widely between studies, reaching values of 7.4-9 kPa [17–19]. Further studies on larger groups of pediatric patients are needed to evaluate the usefulness of TE in assessing the degree of liver fibrosis in pediatric MAFLD, as well as to test its utility in differentiating patients according to the etiology of fibrosis.
Among GDF-15, TSP2, PTX3, ANGPTL8, only TSP2 had significantly higher levels in the group of obese children with MAFLD. TSP2 positively correlated with markers of liver damage such as ALT, AST and GGT. In assessing the correlation with recognized liver fibrosis markers, only a positive correlation with APRI was observed but not with LSM in TE. Similarly, in ROC analysis, despite the statistically significant value of TSP2, its sensitivity was about 50%. Based on our results, TSP2 cannot be considered a useful serum marker of liver fibrosis in children, although it may be a potential marker for assessing liver damage in MAFLD. Data on the involvement of TSP2 in the pathomechanism of NAFLD/MAFLD are scarce. An in vitro model showed that the induction of type I collagen and TSP2 synthesis in hepatic stellate cells (HSCs) is determined by the signaling of transforming growth factor β (TGF-β) [20]. In NAFLD, increased susceptibility of HSCs to TGF-β signaling was observed, leading to disease progression [21]. Further studies are needed to evaluate TSP2 in the mechanism of liver damage in the course of MAFLD.
To the best of our knowledge, serum TSB2 concentrations have not been analyzed among children with any liver disease. In studies involving adult patients with NAFLD, significant correlations between serum TSP2 concentration and the degree of ballooning, steatosis and fibrosis were observed [22]. A study of adult patients with NAFLD and type 2 diabetes showed that serum TPS2 levels were associated not only with obesity-related liver fibrosis, but also with the development of advanced fibrosis in subsequent years [23]. A recent analysis showed the utility of serum TSP2 as a predictive marker of fibrosis stage and hepatocellular carcinoma occurrence in patients with chronic hepatitis after hepatitis C virus (HCV) elimination [24]. Similarly, Iwadare et al. showed that TSP2 levels correlate with liver fibrosis stage in HCV-infected patients [25]. The discrepancies between our results and the studies cited above may be due to the inclusion of patients in different age groups (children vs. adults). In the case of adults, the disease process usually takes longer, which may have an impact on the results obtained. It is also worth noting about the common multimorbidity in adults, as well as the resulting use of pharmacological agents, which may also affect the occurrence of liver fibrosis. Further studies in a larger group of pediatric patients are needed to assess the utility of TSP2 in evaluating liver fibrosis in children.
Simple laboratory tests commonly used in adult patients with NAFLD/MAFLD to assess liver fibrosis include APRI and FIB-4 [26, 27]. In studies conducted in children with liver fibrosis caused by chronic hepatitis B and C virus infections or cystic fibrosis-related liver disease, APRI has shown promise as a marker for predicting and determining the severity of fibrosis [28–30]. In our observations APRI had significant value in differentiating between children with and without MAFLD, suggesting the possibility of fibrosis in this group of examined patients. In a group of 92 children with NAFLD, Mosca et al. comparing various serum tests (including APRI and FIB-4) observed that APRI had better predictive value in diagnosing children with any as well as advanced fibrosis than FIB-4 [31]. Known markers such as APRI are only additional tests to assess liver fibrosis. It seems interesting whether, in combination with new serum markers of fibrosis, APRI can be used in the evaluation of liver fibrosis in everyday clinical practice.
This is the first study in an obese pediatric population to examine a panel of serum markers in combination with other non-invasive predictors of liver fibrosis in children. Our analysis may be an introduction to further analyses in this age group. However, our work has several potential limitations. First, the patients did not meet criteria to perform a liver biopsy [5]; therefore we could not assess the histological grades of fibrosis. Second, the number of patients was too small to draw firm conclusions. The number of patients enrolled in the study was low due to the time span and the monocentric nature of the study. We understand that our results may be subject to errors of omission (type II error), and we did not interpret non-significant statistical results as indicating a true lack of differences.
Conclusions
Our results suggest that TSP2 may be a potential biomarker of hepatocyte injury in pediatric patients with MAFLD. However, GDF-15, TSP2, PTX3 and ANGPTL8 were not found to be effective non-invasive markers of liver fibrosis in children. Further research is needed on the use of TSP2 in the diagnosis and monitoring of MAFLD in pediatric patients.






