Introduction
The thyroid gland is one of the most important organs of the neuroendocrine system, exerting the widest multi-vector spectrum of metabolic effects on organ systems and their functional activity. According to the results of numerous clinical and experimental studies, thyroid hormones have a critical effect on the processes of embryonic development, and cell and tissue differentiation, and they take an active part in maintaining homeostasis in the body, carrying out immune response reactions and apoptosis [1]. The thyroid gland also has a significant effect on the morphofunctional state of the organs of the female reproductive system, the formation and regulation of the ovarian menstrual cycle, as well as the age-related decline of the generative and endocrine function of the ovaries [2]. According to leading experts, the main influence of thyroid hormones occurs at the second and third levels of functional regulation of the female reproductive system.
It is known that at present the scientific community has accepted the concept of a 5-level structural- functional model of regulation of the female reproductive system, which quite reasonably and conclusively explains most neuroendocrine interactions in the female body [3]. According to these ideas, the cerebral cortex and limbic system (hippocampus and amygdala) are recognised as the first (highest) level of regulation. These structures of the central nervous system change the activity of neuroreceptors by influencing them with neurosteroids and transmitter substances (neuropeptides, amino acids, monoamines), and thereby modulate functional and metabolic effects in the brain, influence the processes of myelination and regeneration of myelin sheaths of neurons, as well as the production of releasing hormones of the hypothalamus, which are involved in the implementation of the second level of regulation of the female reproductive system [4].
Numerous experiments have shown that the hypothalamus is the highest vegetative centre, which coordinates the functions of all endocrine glands and ensures the implementation of physiological adaptation mechanisms at the cellular, tissue, and system levels [5]. The hypothalamus is a part of the diencephalon, a part of the limbic system, and morphologically consists of about 50 pairs of neurosecretory nuclei, divided topographically into 5 large groups (preoptic, anterior, middle, external, and posterior). This complexly organised part of the brain is connected with the cerebral cortex, hippocampus, amygdala, cerebellum, brain stem, and spinal cord by nerve pathways; fibres of sensory neurons from all visceral, taste, and olfactory receptors approach it; the pituitary gland, thyroid gland, adrenal glands, and gonads are under its absolute control [6].
Thus, from a modern point of view, the hypothalamus is considered to be the main neuroendocrine organ, the central link between the autonomic nervous system and the endocrine system. In this regard, at this stage of development of theoretical and experimental medicine, leading neuroendocrinologists identify several neurohumoral systems for regulating metabolism in the body: hypothalamic-pituitary-thyroid, hypothalamic-pituitary-gonadal, hypothalamic-pituitary- adrenal, hypothalamo-prolactin, and hypothalamic-somatotropic (hypothalamus-pituitary-liver) axes [7]. How do these axes intersect, what direct and/or indirect influence do they have on each other? There are no clear answers to these questions yet, but this area of the study is certainly promising and will quite possibly change many stable paradigms, patterns, and approaches in science.
The third level of regulation of the female reproductive system is represented by the pituitary gland.
It should be emphasised once again that the hypothalamic-pituitary system is perhaps the most unique mechanism functioning in the human body, in which the production of pituitary tropic hormones (adrenocorticotropic hormone), thyroid-stimulating hormone (TSH), growth hormone (GH), follicle stimulating hormone (FSH), luteinising hormone (LH), prolactin (PRL), melanocyte stimulating hormone, and lipotropic hormone are controlled and adaptively coordinated by 10 hypothalamic neurohormones. At the same time, releasing hormones and statins of the hypothalamus, showing their stimulating or inhibitory effects, have extremely high biological activity and exceptional specificity of action [8].
It is important to note that the results of modern studies have shown the presence of the so-called pleiotropy (multiple influence) of hypothalamic hormones [9]. For example, thyrotropin-releasing hormone increases the secretion of TSH and PRL, gonadotropin-releasing hormone (GnRH) – FSH and LH, somatostatin inhibits the secretion of TSH and GH, and dopamine has an inhibitory effect on TSH, FSH, LH, and PRL, which normally expands the range of metabolic influences and clinical effects significantly [10].
Also, leading experts agree that most of the pathophysiological conditions of the female reproductive system and thyroid diseases are not only interrelated but also often have common or similar pathogenetic mechanisms of initiation [11]. This is explained by the hypothesis about homogenesis of TSH, FST, and LH in the process of ontogenetic development. Thus, these hormones of the adenohypophysis are complex glycoproteins consisting of 2 subunits α- and β-. Their α subunits are identical, but their β subunits are already strictly specific. It is the amino acid composition of the β-chain that determines the selectivity of the hormone’s influence on enzymatic reactions and the implementation of its unique biological effects [12].
As for the regulation of the ovarian-menstrual cycle at the third level, the most significant factors here are the circhoral (pulsating) rhythm of GnRH release and its stimulating effect on the secretion of gonadotropins [13]. It should be noted that the activity and blood levels of FSH and LH are also influenced by the sensitivity of gonadotrophs, the phases of the menstrual cycle, and the current steroid profile in the blood plasma [14]. The last factor, namely the content and ratio of oestrogens, progesterone, and androgens in the blood, regulates the basal secretion of gonadotropins according to the negative feedback principle, and cyclic secretion according to the positive feedback principle. In addition, inhibin B (a glycoprotein hormone that is synthesised by preantral and antral ovarian follicles) and activin (a representative of the transforming growth factor β superfamily of ligands) can act as regulators of FSH production [15].
However, according to some researchers, a real breakthrough in understanding the regulation of the ovarian-menstrual cycle could be the discovery of the kisspeptin neuropeptide in the 2000s, which is produced by neurons located in the rostral preoptic area and the nucleus of the infundibulum of the hypothalamus [16]. Kisspeptin neurons co-express neurotransmitters and neuromodulators such as neurokinin B (NKB) and dynorphins (Dyn). The results of pilot scientific studies indicate a stimulatory effect of NKB and an inhibitory effect of Dyn on kisspeptin. The release of the latter directly enhances the pulsatile secretion of GnRH, which leads to an increase in the secretion of LH relative to FSH and, consequently, to the follicular activity of the ovaries [17]. Thus, the kisspeptin system is currently considered to be a cornerstone regulatory factor responsible for the transmission of homeostatic information to GnRH neurons; therefore, further study of its structural and functional organisation has become a priority task of modern neuroendocrinology [18].
The fourth level of regulation of the female reproductive system is represented by the ovaries, the fifth – by target organs (uterus, vagina, mammary glands, hair follicles, skin, bones, adipocytes), which contain cytosol receptors and therefore have strict specificity for oestradiol, progesterone, testosterone [19]. It is known that 2 main processes occur – folliculogenesis and steroidogenesis – in the ovaries in response to the secretion of gonadotropins and with the participation of growth factors. Maturation of the dominant follicle and ovulation occur under the influence of collagenase, prostaglandins F2α and E2, proteolytic enzymes, oxytocin, relaxin, and inhibin B. However, a decisive influence is exerted by a high (sufficient) level of 17β-oestradiol and the peak emission of LH [19, 20].
With age-related (physiological) decline in the functional activity of the ovaries, trigger changes occur primarily in 2 axes – the hypothalamus-pituitary-ovaries and the hypothalamus-pituitary-thyroid gland [21]. According to modern recommendations and STRAW + 10 criteria, the diagnostic criteria for advancing menopausal endocrinopathy are a persistent increase of FSH, and reduction of anti-mullerian hormone and inhibin B [22]. Taking into account the common origin of FST and TSH in the process of ontogenetic development as well as the spectrum of influences of the thyroid hormones on metabolism described above, it can be assumed that pathological conditions of the thyroid gland can change the course of the menopausal period in a certain way. Therefore, the purpose of our study was to examine the functional state of the thyroid gland in patients with uncomplicated and pathological menopause.
Material and methods
A total of 102 women (49–62 years old) took part in this clinical study. The patients were divided into 2 groups.
Group I included 54 patients who suffered from manifestations of the menopausal syndrome (“hot flashes” to the head and upper body, hyperhidrosis, palpitations, dizziness, headaches, tinnitus, arterial pressure lability, arterial hypertension, feelings of weakness, worn out, anxiety, fibromyalgia, vaginal dryness, burning, itching, dyspareunia, various types of urinary incontinence, discomfort when urinating, and acute and recurrent urinary tract infections).
It should be noted that the duration of the menopausal syndrome in patients in this group was no more than 5 years. This period was not chosen by chance, because the best result of treatment measures is achieved when the time factor from the moment of manifestation of the pathological condition is taken into account.
Group II (control group) included 48 patients whose menopausal period proceeded physiologically. The women did not report significant emotional, mental, and vasomotor disorders as well as symptoms of menopausal genitourinary syndrome.
The menopause rating scale was used to determine the severity of the menopause symptoms [23].
All patients underwent clinical, laboratory, and instrumental examination, according to the recommendations of modern protocols [24]. To assess the functional activity of the thyroid gland, hormonal panel indices were used (TSH, free T3, free T4, according to indications – thyroglobulin, antibodies to thyroid peroxidase – thyroperoxidase antibodies, antibodies to TSH receptors, antibody IgG to TSH receptors). If necessary, ultrasound of the thyroid gland was performed, and the ACR TI-RADS scale was used to identify focal pathology [25].
In addition, the patients of both groups were measured for body mass index (BMI) and blood pressure, an ECG analysis was performed, the results of blood count, haemostasiogram, blood plasma lipid profile (level of triglycerides – TGs, total cholesterol, low-density lipoproteins, very-low-density lipoprotein), and indices of carbohydrate metabolism (fasting blood plasma glucose, oral glucose tolerance test, HOMA-IR index) were assessed.
The examination also included examination of the cervix in the speculum, bimanual examination, and, according to indications, ultrasound of the pelvic organs, mammary glands, and mammography.
To process the study results, methods adopted in biomedical study were used. The material was processed using variational, correlation, and graphical analysis methods using standard and specialised computer programs.
Results
The main stage of the study was the study of the functional state of the thyroid gland, which was assessed by the level of TSH in the blood serum as well as free T4 and T3. The American Thyroid Association recommends their use as universal screening indices that help detect both manifested and subclinical thyroid disorders [26].
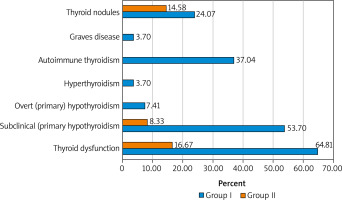
It should be emphasised that during a screening examination of the women who suffered from manifestations of pathological menopause (group I), thyroid dysfunction was detected in 35 (64.81%) patients. At the same time, diagnostic criteria characteristic of subclinical (primary) hypothyroidism (TSH increased, but free T4 within normal limits) were verified in 29 (53.70%) subjects. Manifested (primary) hypothyroidism overt (primary) hypothyroidism (TSH increased, free T4 decreased) was diagnosed in 7.41% of cases, and hyperthyroidism (TSH decreased, free T4, and free T3 increased) in 3.70%.
As for the structure of thyroid diseases, autoimmune thyropathies prevailed in the patients in group I (40.74%). Signs of hypothyroidism due to chronic autoimmune thyroiditis were observed in 20 (37.04%) women, and diffuse toxic goitre in 2 (3.70%). Nodules in the thyroid gland during ultrasound were diagnosed in 13 (24.07%) patients in this group. The ACRTI-RADS scale was used to interpret the detected focal pathology of the thyroid gland. The category TR1 was identified in 4 (7.41%) women, TR2 – in 7 (12.96%), and TR3 – in 2 (3.70%).
During a screening examination of the women in the control group, deviations in the functional state of the thyroid gland were detected 3.9 times less frequently – in 8 (16.67%) patients. Signs of subclinical (primary) hypothyroidism were observed in 4 (8.33%) subjects. It should be noted that among patients whose menopause proceeded without significant clinical manifestations, autoimmune diseases of the thyroid gland were not diagnosed, but the frequency of focal pathology was 14.58%. The category TR1 was diagnosed in 5 (10.42%) women, and TR2 in 2 (4.17%) (Fig. 1).
Fig. 1
Structure of pathological conditions of the thyroid gland in patients who suffer from the menopausal syndrome (group I) and the control group (group II)
According to modern recommendations on providing primary, specialised, and highly specialised care for menopausal disorders, a necessary and important stage of our examination was to determine the patients’ general cardiovascular risk using the SCORE2 scale [27]. The determination of this index is obligatory due to the widespread use of menopausal hormone therapy in treatment programs, which is associated with the risk of venous thromboembolic complications [28].
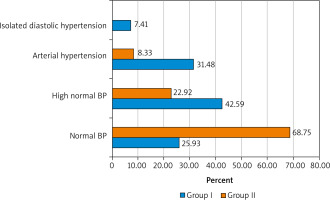
Thus, among the patients under examination in group I, normal blood pressure (120–129 and/or 80–84 mm Hg) was noted only in 14 (25.93%) wo-men, high normal blood pressure (BP) (130–139 and/or 85–89 mm Hg) – in 23 (42.59%), arterial hypertension was diagnosed in 17 (31.48%), with hypertension of stage 1 (uncomplicated) – in 11 (64.71%), stage II (asymptomatic disease) – 5 (29.41%), and stage III (established disease) – in one (5.88%). Stage II hypertension was diagnosed based on the detection of left ventricular hypertrophy on the ECG (R wave in the lead I + S in lead III > 25 mm, R in aVL > 11 mm, R in aVF > 20 mm, S in aVR > 14 mm, R in leads V5 or V6 + S wave in the lead V1 > 35 mm [Sokolov-Lyon index], R in aVL + S in V3 > 20 mm [Cornell voltage index], R in V4, V5 or V6 > 26 mm, highest R + deepest S in the precordial leads > 45 mm, increased rise time of the R wave > 50 ms in the leads V5 or V6, and ST depression and T wave inversion in the “left” leads [signs of LV overload]). Stage III HD was diagnosed due to the presence of objective signs of target organ damage in the patient.
Degree 1 of hypertensive heart disease (BP 140– 159 and/or 90–99 mm Hg) was noted in 14 (82.35%) women suffering from manifestations of the menopausal syndrome, and degree 2 (BP 160–179 and/or 100– 109 mm Hg) – in 3 (17.65%). It should be emphasized that in the first group, 4 patients (23.53%) among patients with arterial hypertension were diagnosed with isolated diastolic hypertension (SBP < 140 and DBP ≥ 90 mm Hg), which, according to the leading cardiologists and therapists, is also a potentially dangerous condition and poses serious health risks without timely correction.
Among the patients in the control group, normal BP values were noted in most women – 33 (68.75%), high normal BP – in 11 (22.92%), and stage I hypertension of first degree was detected in 4 (8.33%) patients. Isolated diastolic hypertension was diagnosed in the women with uncomplicated menopause (Fig. 2).
Fig. 2
Blood pressure in the patients suffering from the menopausal syndrome (group I) and the control group (group II)
BP – blood pressure
The next step of our study was to investigate the lipid profile in patients of the compared groups, namely, to identify the types of dyslipidaemia according to Fridrexen Lipoprotein Patterns Fredrickson Phenotypes. For this purpose, the level of TGs, total cholesterol, low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) were assessed.
Among patients suffering from manifestations of the menopausal syndrome, 9 (16.67%) women were diagnosed with Fridrexen type 4 dyslipidaemia, and 2 (3.70%) were diagnosed with type IIb. Type 4 dyslipidaemia was defined as the presence of normal or elevated total cholesterol, high TGs, normal LDL, and high VLDL. Type IIb dyslipidaemia was diagnosed as a simultaneous increase in the content of total cholesterol, TGs, LDL, and VLDL. These types of dyslipidaemia have a high and very high risk of damage to the peripheral arteries, especially the coronary arteries. Among the patients in the control group, only 2 (4.17%) women had lipid metabolism disorders of the type 4 according to Fridrexen.
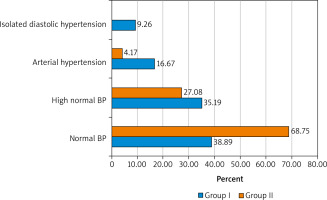
The next stage of our study was the assessment of the main indices of carbohydrate metabolism in the patients of groups I and II. But first, the analysis of the BMI of the women under examination was carried out using the Quetelet index. It was noted that excess weight was observed in 61.11% of women suffering from the menopausal syndrome (overweight (BMI 25–29.9 kg/m2) – in 19 (35.19%), obesity of degree I (BMI 30–34.9 kg/m2) – in 9 (16.67%), obesity of degree II–III (BMI > 35.0 kg/m2) – 5 (9.26%)
The patients with uncomplicated menopause were observed to have opposite trends. Normal body weight (BMI < 25.0 kg/m2) was observed in most of the women – in 33 (68.75%), overweight (BMI 25–29.9 kg/m2) in 13 (27.08%), obesity of the degree I (BMI 30–34.9 kg/m2) in 2 (4.17%), while obesity of degree II–III (BMI > 35.0 kg/m2) was not detected.
To assess carbohydrate metabolism, the recommendations of the American Diabetes Association (2019) were used.
Normal fasting plasma glucose (FPG less than 5.6 mmol/l) was observed in 26 (48.15%) women of group I, prediabetes (FPG 5.6–6.9 mmol/l) – in 22 (40.74%), diabetes mellitus (FPG ≥ 7.0 mmol/l) was diagnosed in 6 (11.11%) in the control group (77.08%) FPG was less than 5.6 mmol/l, indices characterising prediabetes – in 22.92%. No laboratory signs of diabetes mellitus were identified in this group of examined patients. The average fasting blood serum glucose levels in women of group I were 5.89 ±0.25 mmol/l, and in the control group 5.02 ±0.32 mmol/l (pI–II = 0.035). The index of insulin resistance (HOMA-IR index) in women of group I was 3.49 ±0.61 mmol/l, and in the control group 1.54 ±0.28 mmol/l (pI–II = 0.005) (Fig. 3).
Fig. 3
Body weight in the patients suffering from the menopausal syndrome (group I) and the control group (group II)
BP – blood pressure
The final stage of our study was the assessment of cardiovascular risk in the patients of both groups. Calculations have shown that among patients suffering from the menopausal syndrome there is a moderate risk (≥ 1 and < 5%) in 7 (12.96%) women, and high (≥ 5 and 10%) in 2 (3.70%), i.e. the possible use of menopausal hormone therapy in these clinical situations should be limited and even contraindicated. In the patients with uncomplicated menopause, the majority (93.75%) had low cardiovascular risk (< 1%), and it was moderate in 3 (6.25%) women.
Discussion
One cannot but agree with the statement of many researchers and practitioners that the menopausal and postmenopausal periods are a kind of reflection of a woman’s general somatic health. The physiological menopausal transition is possible only with a high (sufficient) level of adaptive metabolic reactions, in the implementation of which thyroid hormones are directly involved. Of course, the thyroid gland is an important neuroendocrine regulator of homeostasis and a significant part of neuroendocrine cooperation in the body [29].
According to the results of multicentre studies, thyroid dysfunction is the most common endocrinopathy in women after 40–45 years of age [30]. An important knowledge for a practicing physician is that the manifestation of thyroid diseases often occurs during the perimenopausal period and is due to the need to activate adaptive processes. Some similarity between the symptoms of menopausal syndrome and thyroid dysfunction creates conditions for masking the clinical manifestations of both pathological conditions, which can lead to their incorrect clinical interpretation and ineffective management.
The results of our study showed that the thyroid gland has a significant impact on the course of menopause. When examining women who suffered from manifestations of pathological menopause, thyroid dysfunction was detected in more than half of the patients (64.81%), while the hypothyroid state was most frequently diagnosed (61.11%). Among thyroid diseases, chronic autoimmune thyroiditis prevailed (37.04%), and diffuse focal and focal changes were observed in 24.07% of cases. In patients with uncomplicated menopause, thyroid pathology was diagnosed 3.9 times less frequently. No autoimmune thyropathies were identified among the patients in the control group; focal pathology (category TI-RADS1 and TI-RADS2) was detected in 14.58% of cases during ultrasound.
During our clinical study it was also found that the patients suffering from manifestations of the menopausal syndrome had a high normal blood pressure, and arterial hypertension was diagnosed in 74.07% of cases, the level of total cholesterol in the blood was elevated in 20.37% of patients, excess weight was observed in 61.11% of those examined, and type 2 diabetes was diagnosed in 11.11% of cases. These additional clinical laboratory studies were carried out to determine the category of cardiovascular risk (the risk of thromboembolic complications) – an important prognostic index that must be taken into account when drawing up a treatment program, especially in patients who require menopausal hormonal therapy [31]. It should be noted that in 16.67% of patients suffering from the menopausal syndrome, the risk of developing atherosclerotic (fatal) cardiovascular complications is assessed as moderate and high.
In our opinion, the thyroid dysfunction, accompanied by hypothyroidism, can be considered as a risk stratification factor or biochemical marker for the development of pathological menopause. It must be emphasised that the results of this study do not prove a cause-and-effect relationship in the formation of the menopausal syndrome. This requires a meta-analysis of studies, and a longer and more detailed study of this issue. However, the information obtained is useful for clinicians and guides practitioners to conduct a more expanded diagnostic search, which will allow them to select personalised and, most importantly, pathogenetically targeted drug therapy. This is consistent with other researchers whose works have shown that adequate correction of thyroid homeostasis helps to stabilise the clinical manifestations of oestrogen-dependent conditions in women.
Conclusions
Considering the multifactorial nature of the development of the menopausal syndrome, the clinical and endocrinological features of its formation, and the presence of functional relationships between the hypothalamic-pituitary-thyroid and hypothalamic-pituitary- ovarian systems, it is rational to include assessment of the thyroid hormonal profile in the program of routine examinations of patients with menopausal disorders. This will enable a timely diagnosis of thyroid diseases and the prescription of effective comprehensive drug correction of the menopausal syndrome.











