Introduction
Hepatocellular carcinoma (HCC) is among the most prevalent malignancies and the second leading cause of cancer-associated death at the global level [1, 2]. While hepatectomy is the optimal approach to HCC patient treatment, 30% or fewer patients diagnosed with HCC are eligible for curative treatment owing to advanced disease staging, limited liver reserve capacity, or lack of access to liver transplantation [3–5].
In patients with inoperable HCC, transarterial chemoembolization (TACE) is commonly employed as an alternative therapeutic modality that is recommended as a first-line management approach for stage B patients under the Barcelona Clinic Liver Cancer (BCLC) guidelines [6–8]. TACE and subsequent percutaneous ablation (PA) can effectively improve HCC patient outcomes [5], and patients who undergo TACE combined with PA reportedly exhibit significant improvements in progression-free survival (PFS) (45 vs. 4 months) and overall survival (OS) (59 vs. 16 months) relative to those patients treated via TACE alone [5]. According to the BCLC guidelines, PA is primarily used to treat HCC patients with stage A disease [8]. In individuals exhibiting tumors proximal to the liver surface, diaphragm, gallbladder, or major vessels, however, PA is not an appropriate approach [9]. In these patients, some studies have employed radioactive seed insertion (RSI) as an alternative therapeutic strategy [10, 11]. To date there have only been a relatively small number of studies focused on the combination of TACE and RSI as a means of treating HCC.
Aim
This study was designed with the goal of comparing the clinical efficacy and long-term outcomes associated with TACE plus RSI to those associated with TACE alone.
Material and methods
Patient selection
The Institutional Review Board of our institute approved this study and waived the need for written informed consent. From January 2018 to December 2021, a total of 80 patients with HCC underwent treatment in our center via either TACE plus RSI (n = 39) or TACE alone (n = 41). To be eligible for study inclusion, patients had to (a) be diagnosed with HCC, (b) have inoperable disease or have declined to undergo hepatectomy, (c) have 3 or fewer tumors, and (d) not be candidates for PA. Patients were excluded if they (a) had undergone any prior treatments for HCC, (b) exhibited diffuse HCC, or (c) exhibited disease of BCLC stage C or higher. Initially, all patients in this study were scheduled for combined treatment (TACE + RSI) before TACE. However, 41 patients did not receive RSI because of their poor family economic condition and they only received TACE.
Diagnosis
HCC was diagnosed based on contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) results exhibiting a pattern of enhancement consistent with arterial hypervascularity and venous/delayed phase washout [12]. In cases where these typical imaging findings were not observed, patients underwent percutaneous biopsy. HCC patients were not candidates for PA in cases where the tumor was < 5 mm from adjacent organs [9].
TACE treatment
TACE was performed under local anesthesia with fluoroscopic guidance using a combination of 5-fluorouracil (150 mg), mitomycin (10 mg), epirubicin (50 mg), and lipiodol (10–20 ml).
Combined TACE and RSI treatment
TACE was performed prior to RSI in all cases. Prior to TACE, abdominal CT images were transferred into the treatment-planning system (TPS; Fei-Tian, Beijing, China) in order to approximate the number of radioactive 125I seeds necessary and to optimize the distribution of those seeds. After manually defining tumor contours on axial CT slices, the TPS system was used to establish the gross tumor volume (GTV). The radiation dose for all patients was in the range 100–140 Gy, with at least 90% of the GTV receiving the selected dose of radioactivity.
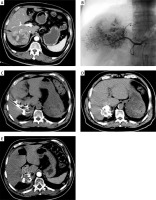
All RSI procedures were performed 14 days after TACE with guidance from a 64-row CT instrument (Siemens, Erlangen, Germany) (Photo 1). Multiple 18G puncture needles were inserted into the tumor in accordance with the design of the TPS system, after which 125I seeds were introduced into the tumor at a spacing of 5–10 mm based on the established treatment plan. The needles were withdrawn so that these seeds were deposited in the needle pathways. A further round of CT scanning was used to confirm seed distributions. After the insertion of 125I seeds, we routinely used hemostatic drugs for 1 day to prevent bleeding.
Photo 1
Procedures of combined TACE and RSI treatment for HCC. A – Preoperative contrast-enhanced CT showed the HCC (arrow) at the right lobe. B – TACE treatment for HCC. C, D – Procedures of CT-guided RSI after TACE. E – Post-operative contrast-enhanced CT indicated that the treatment response was CR
Follow-up
Patient follow-up was performed at 1 and 3 months after treatment, and every 3 months thereafter. Follow-up was performed until December 2022. Follow-up analyses included routine blood analyses, liver function tests, MRI or contrast-enhanced CT imaging, and analyses of α-fetoprotein (AFP) levels. If evidence of MRI/CT enhancement in the treated tumor area was observed, TACE was repeated.
Definitions
Treatment responses at 1 month following treatment were evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria [13]. For details regarding the criteria used to define complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), see Table I. PFS was defined as the period of time between treatment and disease progression or death, while OS was the period of time from treatment to death or most recent follow-up.
Table I
Modified response evaluation criteria in solid tumors for hepatocellular carcinoma
Statistical analysis
SPSS 16.0 (SPSS, Inc., IL, USA) was used for all data analyses. Results were reported as means ± standard deviations when normally distributed and otherwise presented as medians (Q1; Q3), and were respectively compared with t-tests and Mann-Whitney U tests. When comparing categorical variables, χ2 tests or Fisher’s exact test were employed. Kaplan-Meier curves and log-rank tests were used for comparisons of OS and PFS rates. Multivariate Cox regression analyses were used to identify factors independently related to survival outcomes. The factors with p < 0.1 on univariate Cox regression analysis were included in the multivariate Cox regression analysis. P < 0.05 was used as the cut-off to define statistical significance.
Results
Patient characteristics
The baseline characteristics of patients in both groups were comparable (Table II). In total, 1790 125I seeds were placed in 39 patients in the combination group (45.9 seeds/patient). No treatment-related complications were reported for any patients.
Table II
Baseline data of the included patients
Treatment response rates
Treatment response rates in both groups are summarized in Table III. Patients who underwent combination treatment exhibited significant improvements in both CR (59.0% vs. 22.0%, p = 0.001) and total response (92.3% vs. 58.5%, p = 0.001) rates relative to patients treated via TACE alone.
Treatment-related toxicity
There were no significant differences between the two patient groups with respect to the most common forms of treatment-related toxicity, which included fever (38.4% vs. 39.0%, p = 0.959), vomiting (33.3% vs. 43.9%, p = 0.332), and myelosuppression (28.2% vs. 29.3%, p = 0.916) (Table IV).
Progression-free survival
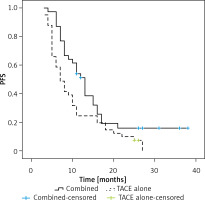
Patients in the combination treatment group exhibited a significant increase in median PFS relative to patients who underwent TACE alone (13 vs. 7 months, p = 0.019, Figure 1). The respective 1- and 3-year PFS rates of patients who underwent combination treatment were 51.2% and 16.0%, whereas for patients who underwent TACE alone, these respective rates were 24.4% and 0.0%.
In univariate Cox regression analyses, AFP > 400 ng/ml (p = 0.048) and undergoing TACE alone (p = 0.028) were associated with a risk of shorter PFS. In a multivariate analysis, only undergoing TACE alone remained independently associated with the risk of shorter PFS (p = 0.033, Table V).
Table V
Predictors of progression-free survival
Overall survival
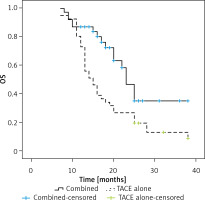
Over the course of follow-up, 17 and 37 patients in the combination and TACE only treatment groups died, respectively. Individuals in the combination treatment group exhibited significantly longer median OS relative to the TACE only treatment group (23 vs. 15 months, p = 0.005, Figure 2). The respective 1- and 3-year OS rates of patients who underwent combination treatment were 87.2% and 35.1%, whereas for patients who underwent TACE alone, these respective rates were 73.2% and 13.0%.
In univariate Cox regression analyses, risk factors for lower OS included being male (p = 0.089), exhibiting an AFP level > 400 ng/ml (p = 0.049), having a larger tumor diameter (p = 0.004), and undergoing TACE alone (p = 0.008). In a multivariate regression analysis, only larger tumor diameter (p = 0.016) and TACE alone (p = 0.029) were associated with significantly reduced OS (Table VI).
Table VI
Predictors of overall survival
Discussion
While TACE remains a key component of HCC patient care under BCLC guidelines, many other therapeutic approaches can be combined with TACE to enhance its efficacy, including RS, PA, and targeted or immunotherapy [7, 11, 14, 15]. PA is commonly combined with TACE [3–7], but it is not well suited to use in cases where HCC tumors are proximal to adjacent organs that may otherwise experience thermal injury [9].
Currently, the insertion of 125I seeds is often employed as an approach to treating various forms of cancer [16–18]. In contrast to more conventional radiotherapy, these 125I seeds offer advantages including their ability to directly contact the internal regions of the tumor, thereby ensuring the prolonged delivery of low-dose radiation in a sustained manner while remaining easy to manipulate [16]. By continuously emitting X-rays and γ-rays, 125I seeds can readily kill target tumor cells and suppress further tumor development [19]. Following 125I seed implantation, significant increases in peripheral blood CD3+ T cell, CD4+ T cell, natural killer cell, and regulatory T cell percentages have been reported in patients with cancer [19]. Circulating C3, C4, IgM, IgA, and IgG concentrations also reportedly rose, suggesting that 125I seeds can provoke robust cellular and humoral immunity [19].
Here, the relative clinical efficacy and associated long-term outcomes were compared for HCC patients who underwent combination TACE and RSI treatment or TACE alone. The CR and total response rates in the combination group were significantly higher than those of patients treated with TACE alone. In the combination treatment group, the total response rate was up to 92.3%, similar to that reported previously following combination TACE and PA treatment (85.9%) [5]. Hence, 125I seeds may facilitate superior therapeutic efficacy for TACE treatment. RSI can also efficiently treat residual HCC tumor cells following TACE [10, 11].
Combining TACE with RSI also contributed to significant improvements in HCC patient OS and PFS relative to TACE alone. The ability of low-dose irradiation derived from 125I seeds to damage and eliminate cycle-sensitive cells and to alter tumor cell distributions can further render HCC cells more sensitive to chemotherapy, resulting in improved long-term therapeutic efficacy [20]. Patients in the combination treatment group exhibited 1- and 3-year OS rates (87.2% and 35.1%) similar to those in a prior report focused on treating HCC with a combination of TACE and PA (85.9% and 32.6%) [21]. As such, RSI may offer a degree of therapeutic efficacy similar to that of PA when treating individuals diagnosed with HCC.
Cox regression analyses confirmed that combined treatment was predictive of the prolongation of patient OS and PFS. Several factors may account for the superior performance of combined therapeutic regimens. For one, tumor embolization can restrict the tumor blood flow [21]. In addition, TACE can generate high levels of ischemia and inflammatory edema in the peritumoral regions, contributing to greater brachytherapeutic benefits upon 125I seed insertion [10, 11]. The 125I seeds were also able to destroy hypovascular HCC tumor regions refractory to precision TACE treatment [10, 11]. A larger diameter was identified as a risk factor for poorer OS, as reported in another similar study focused on treating HCC via a combination of TACE and PA [21]. Larger tumors translate to greater difficulty when attempting to achieve complete necrotic tumor death.
The present results revealed that RSI was not associated with any additional side effects relative to TACE alone. This may be attributable to the use of TPS prior to RSI to select the optimal number and distribution of 125I seeds so as to minimize any radiation-related damage to adjacent healthy tissue.
There are some limitations to this study. Notably, this retrospective study is susceptible to a high risk of bias, underscoring the need for future prospective validation of these findings in a randomized controlled trial. Second, these results were derived from a single center, and a larger patient cohort derived from multiple centers will be important when conducting further follow-up research. Third, the majority of enrolled patients had previously experienced hepatitis infections, and so additional analyses will be required to ascertain whether combining TACE and RSI can effectively treat patients with HCC resulting from other etiologies.