The ACURATE neo2 (Boston Scientific, USA) is a supraannular, self-expanding, transcatheter aortic valve implantation (TAVI) device, and the only transfemoral valve whose commissural alignment can be corrected just above the aortic annular plane [1]. The initial Polish experience with its previous generation was published elsewhere [2]. A recent report that the ACURATE neo2 was not non-inferior to FDA-approved TAVI devices in the US-based IDE trial [3] led to release of detailed procedural guidelines, mainly focused on prevention of underexpansion. The latest iteration of this system, the ACURATE Prime, was developed to improve performance of the device by increasing the opening force and to improve the ability to achieve commissural alignment [4] (Figures 1 A, B). Furthermore, with the reconfigured positioning marker and rapid final release feature, it was supposed to provide the user with increased precision of positioning, which was previously described as a predictor of a need for permanent pacemaker implantation after transcatheter aortic valve implantation [5]. The size matrix of the ACURATE Prime was expanded to include size XL, with a nominal diameter of 29 mm, which makes it possible to treat patients with an aortic annular perimeter of up to 91 mm [4]. We report the initial Polish experience with transfemoral implantation of the ACURATE Prime valve.
Figure 1
Transcatheter aortic valve implantation (TAVI) procedure. A – Correct pre-implantation valve orientation: in 3-cusp coplanar view white arrow indicates 1-1-1 configuration of commissural markers, while in cusp-overlap view 2-1 configuration is confirmed. B – Aortic annulus anatomy based on computed tomography (CT) angiography. C – Tricuspid aortic valve with calcification of leaflets. D – Final valve position and structure after release with fluoroscopic confirmation of perfect commissural alignment in a CT predetermined, right and left coronary cusp overlap view
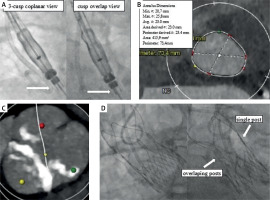
A 65-year-old male patient was admitted for elective TAVI. The heart team deemed the patient unsuitable for open-heart surgery due to advanced pulmonary obstructive pulmonary disease, marked obesity and bilateral occlusion of internal carotid arteries. At admission, he reported dyspnea class III according to the New York Heart Association (NYHA) functional classification. Transthoracic echocardiography (TTE) confirmed severe stenosis of the tricuspid aortic valve with a mean gradient of 48 mm Hg, while left ventricular function was preserved. Computed tomography showed suitable femoral access and perimeter-derived annular diameter of 23.4 mm (Figure 1 C). The valve was confirmed tricuspid with more pronounced calcification of a non-coronary leaflet (Figure 1 D). The ACURATE neo2 valve with its expandable delivery 14F sheath, iSleeve, is a preferred device for patients with obesity in our institution. It was decided to use the ACURATE Prime to treat this patient.
The procedure followed a standard minimalistic approach. Following guidelines for a predilatation balloon choice, being one millimeter smaller than the perimeter-derived annulus diameter, we performed predilatation using a 22 mm VACS-III (Osypka, Germany) semi-compliant balloon over a pre-shaped Safari S (Boston Scientific, USA) guidewire. After insertion into the ascending aorta, a slight rotational correction of valve orientation above the aortic annulus was necessary to correct for commissural alignment. The ACURATE Prime M, with a nominal size of 25 mm, was then implanted in a standard stepwise approach. As angiographic signs of underexpansion were not present, and TTE confirmed a mean transvalvular gradient of 6 mm Hg and only trace perivalvular leak (PVL), postdilatation was not performed. Perfect commissural alignment was documented by fluoroscopy. The patient was ambulated the next day after the procedure. As new conduction abnormalities and vascular complications were absent, the patient was discharged home on day 2. At 4 weeks follow-up the patient reported improvement of exercise capacity to NYHA I with no adverse events, while TTE confirmed optimal performance of the valve, with a mean gradient of 8 mm Hg and trace PVL.
Based on our initial experience with the ACURATE Prime valve, we conclude that incorporation of this technology in the practice of a site with ACURATE neo2 valve experience is not challenging and might lead to an improvement of procedural results. We plan to confirm this observation with an investigator-initiated Polish ACURATE Prime Registry. It will prospectively collect procedural and mid-term clinical results of ACURATE Prime implants in two Polish sites involved in a limited market release of this device.








