Introduction
Hepatitis C virus (HCV) is characterized by its small, enveloped virions containing single-stranded positive-sense ribonucleic acid. Presenting a global health challenge, HCV affects approximately 57 million individuals worldwide, resulting in the most severe complications manifesting as cirrhosis and hepatocellular carcinoma [1, 2]. Direct acting antiviral (DAA) therapies for HCV infections, although universal and highly effective, leave the risk of treatment failure in some populations, particularly those with other concomitant liver disorders. Steatotic liver disease (SLD) is a growing clinical and epidemiological problem worldwide. Its occurrence is related to the level of development of a given society, but in an increasingly large area of the world this problem affects various age and social groups [3, 4]. Steatotic liver disease is currently considered the most common, although underdiagnosed, liver disease, usually associated with systemic metabolic disorders. In combination with HCV infection, it may intensify and accelerate the progression of liver disease. Regardless of the intensively discussed and recently modified nomenclature taking into account the complexity of its etiopathogenesis, the basic term remains steatotic liver disease [5].
The aim of this study was to characterize the HCV-infected population with SLD in comparison to the non-SLD population and to evaluate the effectiveness of treatment with DAA.
Material and methods
The study population consisted of 14,346 patients treated with interferon-free regimens enrolled between 2015 and 2022 in the EpiTer-2 database, which is a retrospective, multicenter, real-world experience, a national, investigator initiated program examining therapies for chronic hepatitis C. EpiTer-2 was started by the Polish Association of Epidemiologists and Infectiologists, and with the cooperation of 22 Polish hepatology centers. The present study analyzed patients receiving interferon-free regimens who, regardless of HCV infection, had a diagnosis of steatotic liver disease (SLD) and documented HCV RNA assessment 12 weeks after the end of treatment to determine treatment effectiveness. SLD diagnosis was made based on ultrasound examination. Patient characteristics, HCV infection, severity of liver disease, and response to treatment were analyzed according to the presence or absence of SLD.
The decision to choose antiviral drugs depended on the attending physician based on current national recommendations [6-10] and the reimbursement policy of the National Health Fund. The dosage and duration of treatment were in accordance with the Summary of Product Characteristics of the individual drugs. All patients gave informed consent before starting treatment, following the requirements of the National Health Fund. Clinical and laboratory data were collected retrospectively and transmitted via an online platform operated by Tiba sp. z o.o. by the national regulation on the protection of personal data.
The analysis included demographic data such as age, gender, and body mass index (BMI), as well as information about the HCV genotype, previous treatment attempts, stage of liver disease, and its current course, including decompensation events, diagnosis of hepatocellular carcinoma (HCC), and coinfection with hepatitis B virus (HBV) and human immunodeficiency virus (HIV). The severity of liver disease was assessed by transient elastography (TE), shear wave elastography (SWE), or liver biopsy. The results were converted to the degree of fibrosis F0-4 according to the METAVIR scale, using the recommendations of the European Association for the Study of the Liver (EASL) [11]. In the case of diagnosed liver cirrhosis, the Child-Pugh (CP) score and the Model for End-Stage Liver Disease (MELD) were included in the analysis. HCV RNA measurements were performed by real-time polymerase chain reaction before treatment and 12 weeks after treatment to evaluate SVR as a measure of therapy effectiveness.
Statistical analysis
The results were expressed as absolute numbers (percentage) or mean (standard deviation and range). A p-value below 0.05 was considered significant. Statistical significance of the difference in event frequencies between groups was determined by the chi-square test. For continuous variables, the Mann-Whitney U test was employed due to the non-Gaussian distribution. Statistical analyses were performed using the GraphPad Prism 5.1 software (GraphPad Software, Inc., La Jolla, California, United States).
Results
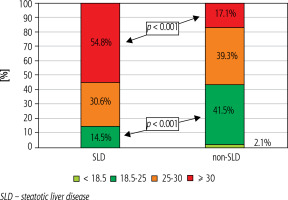
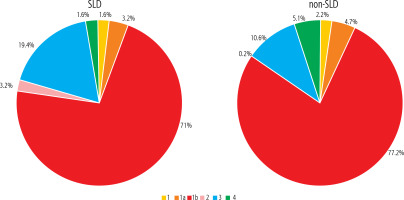
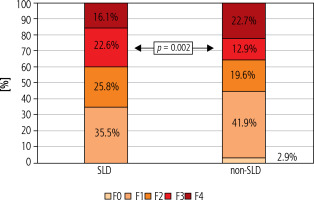
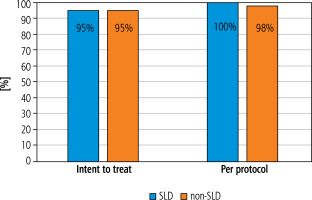
The analysis included 62 patients diagnosed with SLD and 14,284 non-SLD patients. Unlike the non-SLD population, the SLD group was dominated by men (49.5 vs. 53.2%, respectively), but the difference was not statistically significant. The mean age of patients with SLD did not differ from that of the non-SLD population infected with HCV (Table 1). As shown in Table 1, patients with SLD had significantly higher BMI values, were more likely to have concomitant diseases (including diabetes and hypertension), a history of HCC, liver transplantation, HBV co-infection and higher ALT activity. The remaining indicators characterizing both groups did not show statistically significant differences (Table 1). As many as 54.8% of patients with SLD suffered from obesity (BMI ≥ 30), whereas in the non-SLD group they constituted only 17.1%, and this was a statistically significant difference (Fig. 1). Patients with SLD compared to those without steatosis were significantly more often infected with genotypes 2 (3.2% vs. 0.2%, p < 0.0001) and 3 (19.4% vs. 10.6%, p = 0.026). The frequency of other HCV genotypes did not differ, and the dominant one in both groups was genotype 1b (Fig. 2). As shown in Figure 3, the percentage of patients with advanced liver disease (F3/4) was similar in both groups (38.7% vs. 35.6%), but F3 patients were significantly more common in the SLD group. Out of 62 SLD patients included in the analysis, 59 (95.2%) achieved a sustained virologic response (SVR), but after excluding 3 lost to follow-up a response rate of 100% in per protocol analysis was obtained. The corresponding SVR values in the non-SLD population were 95.0% and 97.8%, respectively (Fig. 4). There were no statistically significant differences between groups.
Table 1
Characteristics of HCV-infected patients with (SLD) and without (non-SLD) steatotic liver disease
Discussion
The term steatotic liver disease was introduced in accordance with the multi-society Delphi consensus in June 2023 and replaced the previously used term fatty liver disease [5]. This was not just a simple name change dictated by the stigmatizing nature of the previous terminology and the need to exclude confounding factors during the diagnostic process, but a reflection of the complex causes of the disease problem, the essence of which is the accumulation of excess fats in the liver. It was pointed out that the diagnosis should be based on confirmatory rather than exclusionary criteria. Within SLD, names of subtypes indicate the etiology of the disorder, including metabolic, and alcoholic, but also a “miscellaneous” category that includes steatohepatitis due to HCV infection, among others [5]. However, according to the new approach, multiple etiologies of hepatic steatosis can coexist, and this is the situation for many patients with HCV infection [12, 13].
The pathogenesis of the co-occurrence of liver steatosis and chronic HCV infection is complex and related to host and viral factors, as well as alcohol consumption. Steatosis associated with metabolic host risk factors, such as dyslipidemia, type 2 diabetes, high BMI, and hypertension, frequently occurs in patients infected with non-GT3, while in those infected with GT3, steatosis is mainly driven by the direct cytopathic effect of the virus [14, 15]. Liver steatosis is a common feature in patients with chronic hepatitis C, especially in GT3-infected individuals. In our analysis, the percentage of patients diagnosed with steatosis was not as high as that reported in the literature, but it should be noted that data regarding the frequency of hepatic steatosis in patients with HCV are based on histopathological findings, whereas in our study, patients did not have a liver biopsy, and the diagnosis was made based on ultrasound, which detects steatosis if at least 20-30% of hepatocytes are involved in the process [16, 17]. However, in the studied population, we found a significantly higher percentage of GT3-infected patients among SLD patients compared to the non-SLD group. In the SLD population, we documented a significantly higher proportion of patients with comorbidities, including diabetes, obesity, and hypertension, which are metabolic risk factors for steatotic liver disease, supporting reports from the literature [16, 18, 19]. None of the patients in the analyzed population reported alcohol abuse, but since the patient’s statement was the only source of this information, we cannot exclude the influence of this factor.
Our analysis draws attention to the significantly higher frequency of patients with HBV co-infection among patients with SLD and HCV infection. According to a recently published meta-analysis of 98 studies involving over 48,000 patients, the global incidence of hepatic steatosis in patients with chronic HBV infection is higher than previously estimated, although no effect on the severity of liver disease has been documented [20].
Nevertheless, more frequent occurrence of advanced liver disease, including severe fibrosis and cirrhosis with a higher risk of HCC, has been indicated as one of the possible consequences of the coexistence of liver steatosis and HCV infection compared to HCV infection alone [16, 21]. Although this relationship between co-occurrence of SLD/HCV and cirrhosis was not confirmed in the population we analyzed, the percentage of patients with documented fibrosis at stage F3 in the SLD group was significantly higher, as was the proportion of people after liver transplantation. Comparison of the percentage of patients with a history of HCC also showed an advantage in the group of patients with SLD, although the difference was not statistically significant. Moreover, ALT activity was significantly higher in the SLD group, which may indicate more intense hepatic necroinflammation, confirming the findings from other studies [22-24].
Many reports also indicate another serious consequence of the co-occurrence of fatty liver disease in patients with chronic HCV infection, namely a significantly higher risk of lack of virological response to antiviral therapy [14, 16]. However, it should be noted that these conclusions mainly come from the era of interferon-based therapies [25]. Moreover, the data suggested that it depends on the pathogenesis of steatosis; viral fatty liver disease typical of GT3 may not influence the response to treatment, unlike metabolic steatosis typical of infection with other genotypes [26]. Studies analyzing the impact of hepatic steatosis on response to DAA therapy are lacking, and our study appears to fill this gap by documenting the lack of influence of concurrent hepatic steatosis on the efficacy of antiviral therapy [14]. However, there are reports available confirming the beneficial effect of effective DAA therapy on changes in liver stiffness and steatosis in patients with HCV infection [27].
We did not evaluate the abovementioned long-term effect in our study, which is one of the limitations we are aware of. Another limitation that we must stress is the diagnosis of fatty liver disease based on abdominal ultrasound examination, which is not diagnostic in the case of low severity of the disease process. The retrospective nature of our study, with characteristics typical of this type of design, also results in possible bias and missing data. We also did not objectively analyze alcohol consumption by patients, based on their statement. Another limitation was the disproportion between the compared groups, which made it difficult to compare the results.