Introduction
Hepatitis B virus (HBV) infection remains a significant health problem worldwide despite the availability of an effective vaccine and antiviral treatment, highly potent in suppressing viral replication [1-3]. It is estimated that in 2022 the global prevalence of chronic HBV infection was 3.2%, equivalent to 257 million cases of infection. It can lead to severe health consequences, including liver decompensation, cirrhosis, and hepatocellular carcinoma (HCC), resulting in high morbidity and mortality rates [2]. HBV promotes hepatic damage and cancerogenesis through various mechanisms, including insertional mutagenesis, promotion of host genomic instability and cell disruptions, and activation of oncogenic pathways by viral proteins. In 2019 alone, HBV infections caused 18.2 million global disabilityadjusted life years (DALYs) and 0.7% of all global DALYs. Acute hepatitis, cirrhosis, and liver cancer contributed 8.9%, 59.2%, and 31.9% of DALYs caused by hepatitis B, respectively [4]. Moreover, hepatitis B is estimated to have caused from 487,000 to 630,000 deaths in 2019, mostly from liver cirrhosis and HCC, representing an average 6% increase compared to 2019 and 3% increase compared to 2015 [1]. Available data indicate that deaths attributed to HBV infection accounted for 55% of those from primary liver cancer and 45% of deaths from cirrhosis and other complications of chronic liver disease [5, 6]. This makes HBV infection burdensome for infected individuals but also leads to significant economic costs for the entire population. Liver damage related to HBV infection occurs due to acute or chronic infection, which may be asymptomatic. People with chronic infection can be a major source of HBV transmission because they are often unaware of their status. Only 14% of patients with HBV infection have been diagnosed, and only 8% eligible for treatment were on treatment [2].
To eliminate the HBV infection as a global public threat, the World Health Organization (WHO) endorsed the Global Health Sector Strategy on Viral Hepatitis, which set goals to be achieved in 2030, of reducing new infections by 90%, decreasing mortality by 65% compared to 2015 and, among other objectives, detecting 90% of all chronically infected, and achieving 90% vaccination status of children with three doses of hepatitis B vaccine [7]. Currently, many countries are unable to meet the targets set by the WHO, especially concerning HBV infection diagnosis rates [8, 9]. The lack of national screening programs in 38% of WHO member countries remains a problem, complicating the achievement of HBV infection elimination [10].
HBV infection prevalence shows considerable variability by geographic region, and the epidemiology of the disease reveals differences between countries. Low-developed areas, such as Africa and East Asia, are endemic regions for the virus (high prevalence > 8%). The Mediterranean Basin and Eastern Europe are moderately endemic (2-8%). Highly developed countries, including Western Europe and North America, have a low endemicity and prevalence (< 2%) [2, 9, 11, 12]. According to the most recent data, 14 million people are living with chronic HBV infection in the WHO European Region, while an estimated 4.7 million such individuals live in the countries of the European Union (EU) and the European Economic Area (EEA) [8]. Data estimating the incidence of HBV infection in Poland indicate a 0.4-1.0% prevalence of hepatitis B surface antigen in the population, implying that approximately 339,000 individuals are chronically infected [1, 2, 11]. However, to date, there has been no long-term post-pandemic COVID-19 analysis showing infection detection and death trends due to HBV infection in the Polish population, and this analysis is intended to fill this knowledge gap.
This study aimed to analyze HBV infection and mortality in Poland according to socio-demographic characteristics (gender and age), trends over time (2005-2022), and the potential impact of the COVID-19 pandemic on hepatitis B epidemiology.
Material and methods
Study design
In this population-based national retrospective study in Poland, we analyzed all registered cases of HBV infection from 2005 to 2022 and cases of SARS-CoV-2 infection (COVID-19) from 2020 to 2022. The starting period of the present research was set for 2005 when the case definitions in epidemiological surveillance in Poland were applied, dividing hepatitis into cases of acute hepatitis B (AHB) and newly diagnosed cases of chronic hepatitis B (CHB) [13]. The study included anonymous information on diagnosed cases of HBV infection and deaths, along with socio-demographic characteristics, such as gender and age. Our epidemiological analysis was based on secondary administrative data and did not include any studies with human participants performed by any of the authors or processing of sensitive data. Due to all cases of HBV infection and COVID-19 in Poland being registered and reported for epidemiological surveillance, obtaining informed consent or approval by a medical ethics board was not required under national regulations.
Data sources and HBV infection variables
Information on diagnosed HBV infection and COVID-19 cases was obtained from Epimeld database reports published by the National Institute of Public Health – National Research Institute (NIZP-PZH) [14]. These reports include suspected and confirmed HBV infection cases detected by physicians and laboratories reported to the District Sanitary and Epidemiological Station, where they are verified and recorded by the Polish administrative branch. Data from the Central Statistical Office on registered deaths from HBV infection were also used.
The analysis included diagnosed new HBV infections and deaths, and COVID-19 infections identified using the International Statistical Classification of Diseases and Health Problems, Tenth Revision (ICD-10) codes for acute HBV infection cases (B16), chronic HBV infection cases (B18.0-B18.1) and COVID-19 (U07.1). The HBV infection diagnosis rate was calculated as the number of new cases per 100,000 population in each calendar year and considered the division into gender and age groups (≤ 24, 25-44, 45-64, ≥ 65). To assess the impact of COVID-19 pandemic waves on noted hepatitis B virus infection in 2020-2022, we used monthly information on COVID-19 and HBV infection cases. The total HBV infection and COVID-19 diagnosis rates were calculated as the number of new cases per 100,000 population each month.
We used age-standardized mortality rates (ASMRs) to analyze deaths from HBV infection, calculated using the direct standardization method and the 2013 edition of the European Standard Population as the reference population [15]. ASMR per 100,000 population was determined for each calendar year, considering gender and age groups (≤ 44, 45-64, and ≥ 65).
Statistical analysis
Categorical variables were presented as proportions and compared using a chi-square test. Two-proportion tests with Bonferroni adjustment for multiple comparisons were also used to compare HBV infection cases (diagnosis and death) between individual pairs of subgroups. Wilcoxon rank sum tests were used to compare the distributions of HBV diagnosis and mortality rates between men and women populations.
Joinpoint regression analysis was performed to examine the changes associated with diagnosis and mortality during the last 17 years (2005 to 2021), allowing the detection of significant changes in trends [16, 17]. The applied analytic method automatically identified the number and locations of joinpoints during the observation period, restricting their number to 3, resulting in up to 4 linear segments in the trend line. Estimated linear segments were presented as an annual percentage change (APC). A summary measured over the 2005-2021 period showed the annual average percent change (AAPC), calculated as a weighted average of partial trend APCs. The differences in examined trends of HBV infection between men and women were also examined using the Wald test [17].
Statistical calculations were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corporation, Armonk, NY, USA). The significance level was set at α = 0.05 in statistical tests.
Results
Between 2005 and 2021 in Poland, a total of 36,073 new cases of HBV infection and 870 deaths due to HBV infection were diagnosed, with a higher proportion found in men (constituting 58.4% of diagnosed cases and 68.6% of deaths). Differences in new HBV infections and deaths due to HBV by gender were statistically significant (p ≤ 0.001). Of the total newly diagnosed cases of HBV infection and deaths caused by HBV infection, a significant proportion were types of CHB (92.5% and 83.6%, respectively). Table 1 presents the descriptive statistics for the distribution of HBV infection diagnosis rates and mortality rates. The rate of AHB in men was 0.21 per 105 compared to 0.12 per 105 in women (p > 0.05), and for CHB they were 5.02 per 105 vs. 3.30 per 105 (p ≤ 0.01), respectively. The lowest median diagnosis rates of HBV infection were observed in the age group ≤ 14 years (for AHB in males and females 0.00 per 105, and for chronic HBV infection in males 0.10 and females 0.11 per 105). Also in young women (15-24 years) the median AHB diagnosis rate was 0.00 per 105. The highest diagnosis rates of AHB were found in men aged 25-34 years (0.34 per 105) and in women aged ≥ 65 (0.18 per 105); for CHB, maximum values among men were recorded in the age group 45-54 years (9.37 per 105) and for women in the age group 25-34 (9.32 per 105).
Table 1
Distribution of descriptive statistics for HBV infection by gender and age over the period 2005-2021
There were significant differences in the median rates of mortality due to HBV infection by gender, except for mortality due to acute HBV in the age group ≤ 44 (Table 1). In the years 2005-2021, the median for ASMR of total HBV infection in men was three times higher compared to women (0.12 per 105 vs. 0.04 per 105; p ≤ 0.001). The ASMR value for AHB was low; among men 0.02 per 105, and in women 0.01 per 105 (p ≤ 0.001). The highest values of ASMR for CHB were found in the age group 45-64 years, where for men it was 0.07 per 105 and for women it was 0.02 per 105 (p ≤ 0.001).
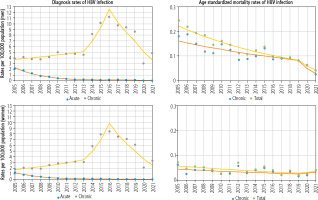
Joinpoint analysis to identify changes in trends in HBV infection diagnosis rates is presented in Table 2 and Figure 1. The results showed pronounced decreasing trends in AHB diagnosis rates in both genders, which appeared mostly at the two joinpoints connecting the three-line trend segment. The overall AHB diagnosis rate in the first trend among men dropped from 2.17 per 105 to 0.27 per 105 (APC2005-2012 –26.45%, ptrend < 0.05) and among women from 1.26 per 105 to 0.17 per 105 (APC2005-2011 –27.72%, ptrend < 0.05). In the subsequent years (second trend), there was a reduction of the total AHB diagnosis rate among men to 0.18 per 105 (APC2012-2019 −8.46%, ptrend < 0.05) and women to 0.06 per 105 (APC2011-2019 −12.86%, ptrend < 0.05). A marked decrease in total AHB diagnosis rate was visible during the third trend after the year 2019 and in the year 2021; among men, the rate decreased to 0.03 per 105 (APC2019-2021 −57.65%, ptrend < 0.05), and among women to 0.02 per 105 (APC2019-2021 −42.10%, ptrend < 0.05).
Table 2
Trends in diagnosis rates of HBV infection by gender and age in Poland over the years 2005-2021 with model-based estimations
The CHB diagnosis rate from 2005 to 2021 appeared mainly at the three points connecting the four-line trend segment. The analysis showed evident trend fluctuations in the CHB diagnosis rate, with a change in trend direction in 2015/2016 (Table 2 and Figure 1).
The total diagnosis rate of CHB during the first trend increased among men from 3.91 per 105 to 4.58 per 105 (APC2005-2013 +3.89%, ptrend < 0.05) and among women from 1.81 per 105 to 3.05 per 105 (APC2005-2013 +9.02%, ptrend < 0.05). In the second trend, the rate of total CHB diagnosis increased significantly in both genders: among men to 11.20 per 105 (APC2013-2016 +34.90%, ptrend < 0.05) and women to 8.43 per 105 (APC2013-2016 +41.35%, ptrend < 0.05). In 2016 a change was observed in the direction of the third trend for negative, and the diagnosis rate of CHB decreased among men to 8.60 per 105 (APC2016-2019 –17.74%, ptrend > 0.05) and for women to 6.12 per 105 (APC2016-2019 –20.13%, ptrend > 0.05). However, the fourth trend after 2019 showed a sharp reduction in the diagnosis rate of CHB among men to 4.82 per 105 (APC2019-2021 –26.94%, ptrend < 0.05) and among women to 3.30 per 105 (APC2019-2021 –28.96%, ptrend < 0.05).
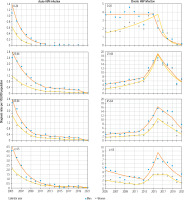
Changes in trends in HBV infection diagnosis rates by age are presented in Table 2 and Figure 2. In age groups ≤ 24 years for AHB diagnosis rates, there was only a first trend, which decreased among men from 2005-2012 and for women from 2005-2009 (for both genders ptrend < 0.05). However, among men in the 25-44 and 45-64 age groups, all three segments of the AHB diagnosis rate trend were reduced (ptrend < 0.05), with dramatic declines in the third trend (for age 25-44 it was –67.06%, and for 45-64 it was –54.62%). Among women in the 25-44 and 45-64 age groups, the first and second trends decreased dynamically (ptrend < 0.05), and there were decreases in the third trend in both age groups, but the changes were not statistically significant (ptrend > 0.05). Among men in the oldest age groups ≥ 65 years, only the first segment of the AHB diagnosis rate decreased significantly (ptrend < 0.05); the second trend was positive but did not reach statistical significance (ptrend > 0.05), and the third trend was negative but did not reach statistical significance (ptrend > 0.05). Among women in the age group ≥65 years, the first trend of the AHB diagnosis rate decreased dynamically (ptrend < 0.05), the second line segment showed no significant change (ptrend > 0.05), and there was a clear, sharp decreasing trend of –49.01% (ptrend < 0.05) after 2019.
There were no statistically significant differences in all linear trends in the AHB diagnosis rate between men and women throughout the analysis period 2005-2021 (p > 0.05). This suggests that the dynamics of the AHB trend were similar in men compared to women. For example, the total AHB diagnosis rate AAPC2005-2021 among men was –19.90% and women –19.78%, p = 0.920 (Table 2).
Taking into account the age groups, the group ≤ 24 years for the diagnosis of CHB for both genders demonstrated two trend segments with a change in the direction of the trend at the turn of 2015/2016 from the positive first trend (ptrend > 0.05) to the negative second trend (in men APC2015-2021 –42.83%, ptrend < 0.05, in women APC2016-2021 –55.64%). In the 25-44 and 45-64 age groups, the changes in both genders were quite similar: the first and second trends of CHB were generally positive. However, the second trend increased very dynamically until 2016, especially in men aged 25-44 (APC2013-2016 35.07%, ptrend < 0.05) and women aged 45-64 (APC2013-2016 53.13%, ptrend < 0.05). In the following years, these trends rapidly turned negative (in the age group of 25-44 in men APC2016-2019 –18.90%, ptrend < 0.05, in the age group 45-64 in women APC2016-2019 –17.40%, ptrend < 0.05).
After 2019, the negative CHB trend continued, but the rate of decline increased (in the age group 25-44 in men APC2019-2021 –26.88%, ptrend < 0.05, in the age group 45-64 in women APC2019-2021 –28.13%, ptrend < 0.05). The third trend of CHB was negative in women aged 25-44 (APC2016-2021 –22.02%, ptrend < 0.05) and men aged 45-64 (ptrend > 0.05). In men in this age group the fourth trend also did not reach statistical significance. In the oldest age group ≥ 65 years, in both genders the first trend was negative until the turn of 2012/2013, when the increase in the CHB diagnosis rate began, lasting until the turn of 2015/2016. In the following years, in men after 2016, there was a significant decrease (APC2016-2021 –20.52%, ptrend < 0.05), and in women in the period 2015-2018, the trend stopped, and after 2018 it decreased dynamically (APC2016-2021 –28.09%, ptrend < 0.05) (Table 2 and Figure 2).
During the analyzed period 2005-2021, there were statistically significant differences in linear trends of CHB diagnosis rate between men (AAPC = 4.17%) and women (AAPC = 7.22%), p = 0.001. Differences in trends for CHB concerned the age group ≤ 24, in which AAPC2005-2021 for men decreased faster compared to women (−16.53% vs. −12.77%, p = 0.032); and for the age group 45-64 years, there was an increase (9.87% vs. 10.11%, respectively, p = 0.001) (Table 2).
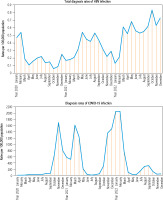
During the COVID-19 pandemic in the following months of 2020-2022, the rate of total HBV infection diagnosis changed dynamically along with the waves of the pandemic (Fig. 3).
The lowest values recorded in October 2020 (0.06 per 105) and January 2022 (0.13 per 105) coincided with the peaks of the COVID-19 waves in November 2020 (1688 per 105), and January and February 2022 (diagnosis rates 2060 per 105 and 2064 per 105 respectively). On the other hand, low COVID-19 diagnosis rates in the period from May to September 2021 (range 8-213 per 105), May to June 2022 (range 20-30 per 105) and November to December 2022 (range 33-42 per 105) generally coincided with high total diagnosis rates of HBV infection in May and August 2021 (range 0.42-0.54 per 105), May 2022 (0.68 per 105) and October 2022 (0.83 per 105).
Fig. 3
Changes in burden of hepatitis B virus (HBV) infection by month in the years 2020-2022 and COVID-19 pandemic waves
The period of the COVID-19 pandemic 2020-2022, taking into account the division into months, brought large changes in the value of total HBV infection diagnosis rates compared to 2019 (Table 3). In the first year of the pandemic, in March 2020 (vs. March 2019), there was a dramatic reduction in the total diagnosis rates of HBV infection of –74%, while in the summer of 2020 there was a reduction of –68% (vs. summer of 2019). During the pandemic, the largest change in the diagnosis rates of HBV infection occurred in October 2020 (vs. October 2019) and was –91%. After the next ten months, the rate of HBV was lower by –4% in August 2021 vs. August 2020, and in the next period from September 2021 to January 2022, the decrease in the rate of HBV infection significantly deepened (from –19% to –80%). In August 2022, the value of the HBV rate equaled the level in August 2019 (0% change), and in September 2022 (vs. September 2019), positive values appeared (+15%), and by December 2022, they increased dramatically to +46%.
Table 3
Changes in total diagnosis rates of HBV infection by months during the COVID-19 pandemic (between 2020 and 2022 in compare to 2019)
The analysis of total mortality due to HBV infection between 2005 and 2021 among both genders shows the changes with one joinpoint connecting two line segments of the trend (Table 4 and Figure 1). Among men in the years 2005-2019, the value of ASMR for total HBV infection decreased by 6.74% (ptrend < 0.05) from 0.24 per 105 to 0.08 per 105. In subsequent years until 2021, the decrease was insignificant (ptrend > 0.05), and ASMR decreased to 0.04 per 105. In the case of CHB in men, the value of ASMR in the years 2005-2019 decreased significantly to 4.61% from 0.18 per 105 to 0.08 per 105, and by 2021 there was still a decrease in ASMR to 0.03 per 105, but this change was not statistically significant. Among women, there was a decrease of ASMR for total HBV infection by 4.83% in the years 2005-2019, from 0.07 per 105 to 0.02 per 105, while in 2021, there was a slight increase to 0.04 per 105 (ptrend > 0.05). The trend was insignificant for ASMR of CHB among women throughout the analyzed period.
Table 4
Trends in age-standardized mortality rates due to HBV infection by gender in Poland in the years 2005-2021 with model-based estimations
Taking into account the entire analyzed period of 2005-2021, the decrease in mortality was significant in both genders, but changes were faster in men compared to women (by –8.07% and –3.99%, respectively). Hence, there was a statistically significant difference in dynamic trends in mortality due to total HBV infection between genders (p = 0.027). In the case of mortality due to CHB in the period 2005-2021, the trend in both genders was negative (ptrend > 0.05), and also there was no statistically significant difference in dynamic trends of ASMR for CHB between genders (p > 0.05).
Discussion
In our study on HBV infection epidemiology in Poland from 2005 to 2021, we found a decrease in AHB infection in both genders, particularly dynamic in the period 2005 to 2009/2012 and during the COVID-19 pandemic. This sharp decrease in the initial period suggests that favorable changes began much earlier and were already visible in 1996. At that time, the incidence of HBV infection in Poland fell below the level reached in the EuroWHO countries, which was related to the improvement of sterilization in hospitals, as well as the implementation of vaccinations among healthcare workers and patients referred for surgery [18]. However, it should be emphasized that the downward trend in AHB infection in 2005-2012 in Poland was caused by the introduction of vaccination against HBV infection in the obligatory calendar of preventive vaccinations for newborns in 1994-1996. The findings of our study revealed that the small number of AHB infections in the age group of 0-24 years is evidence of the effectiveness of vaccination, which was also observed in other countries where population vaccination programs were introduced [20, 21]. In addition, another benefit of vaccination is the effect of reducing mortality due to HBV infection.
The trend for the diagnosis rate of CHB infection during 2005-2021 showed a fluctuation from an upward trend with low dynamics, turning into a fast positive trend, and then a clear change in the trend direction to negative. The initial upward trend in CHB may be the result of the high incidence in Poland in the 1980s and 1990s [18]. However, the dynamic increase in CHB infection after 2013 resulted from legislative changes that enabled a more complete assessment of the epidemiological situation of CHB infection [13]. In 2014, an extended HBV case definition based solely on laboratory criteria was introduced, allowing the registration of cases based on a single positive hepatitis B surface antigen test result. In addition, also from 2014, cases of hepatitis B were reported by laboratories in which markers of HBV infection were detected [13]. On the other hand, the sudden turn of the trend to negative at the turn of 2015/2016 can be interpreted as the result of mapping the negative CHB curve from the dynamic decrease in AHB infection in the earlier period. It is worth emphasizing that the population aged 15-19 at that time of the study was vaccinated in the neonatal period.
The current study is an important source of information regarding changes in the hepatitis B burden during the COVID-19 pandemic. During this time, there was a spectacular reduction in infection rates of AHB and CHB. This change was caused by limitations in the field of prophylaxis, diagnostics, and routine patient care, and these problems in the Polish population were more pronounced than in other EU countries [22-24]. Unfortunately, the difficulties caused by the pandemic may lead to the loss of existing efforts to eradicate HBV. It should also be noted that starting from September 2022, the diagnosis rate of HBV infection exceeded the levels achieved in 2019, so it requires careful and continuous monitoring. This result is not only the effect of the pandemic but the possible impact of the migration crisis that occurred in Poland in 2022 due to the war in Ukraine. The mass influx of refugees from neighboring countries with higher seroprevalence and insufficient vaccination of children [1, 2, 25] requires the development of guidelines for screening HBV infection in the migrant population to prevent the epidemiological situation from worsening [3, 26].
The main strength of this study is focusing on long-term trends for hepatitis B based on data for the whole population of Poland. Using an innovative joinpoint regression approach, we examined 17-year trends (2005-2021) of acute and chronic HBV infections as well as mortality trends due to HBV infection, which have not been previously explored. Our study may have certain limitations related to the imperfections of the HBV infection registration system consisting of incomplete reporting of detected cases of infections and deaths. Similar registration problems in the epidemiological surveillance systems also occurred in other countries [9, 27]. In addition, the epidemiological situation of HBV infection in Poland may be underestimated due to difficulties in access to medical care and insufficient diagnostics.
Conclusions
The COVID-19 pandemic has clearly reduced the frequency of HBV infection diagnoses in Poland, especially acute cases. However, trends of hepatitis B infection require further monitoring of the impact of the pandemic on the viral hepatitis response. The results suggest that introducing a national screening program and improving linkage to care are necessary. Developing guidelines for screening HBV infection is also important, especially among the migrant population.