Introduction
Breast cancer (BC) remains the most frequently diagnosed cancer in females [1]. Triple-negative breast cancer (TNBC) accounts for approximately 15–20% of all breast carcinomas and is immunohistochemically characterized by the absence of three receptors: estrogen receptor (ER), progesterone receptor (PR) and human growth factor receptor (HER2) [2]. The prognosis for TNBC is worse than that for luminal subtypes. Recurrences and cases of death are observed within 3–5 years after a diagnosis [3, 4]. Chemotherapy has become the main approach for the treatment of TNBC because, as studies in clinical practice have shown, TNBC is more responsive to chemotherapy than any of the other molecular subtypes [5, 6]. Neoadjuvant chemotherapy (NAC) allows for the reduction of the tumor volume and regional lymph nodes, which can facilitate more options for surgical treatment. Complete pathologic regression allows one to conduct breast-conserving treatment (BCS). However, TNBC commonly harbors BRCA mutations, so mastectomy is the preferred surgical treatment for patients with mutations [7]. In the last two decades, both nipple-sparing mastectomy (NSM) and skin-sparing mastectomy (SSM) with immediate reconstruction with a prosthesis or an expander have been used in the surgical management of nonmetasta-tic breast cancer patients. According to the literature, outcomes of treatment with NSM, SSM, and modified radical mastectomy (MRM) are similar, but, significan-tly, subcutaneous mastectomies preserve the patient’s body shape and can increase the quality of life [8, 9]. The introduction of sentinel lymph node biopsy (SLNB) into the surgical treatment has replaced the routinely utilized axillary dissection and made it possible to avoid the complications of axillary lymphadenectomy. NSM or SSM can be connected with sentinel lymph node biopsy in patients with clinically negative lymph nodes. The aim of our retrospective study was to analyze the outcomes of combined treatment of patients with TNBC treated with subcutaneous mastectomy.
Material and methods
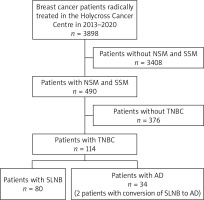
Detailed information on data verification is presented in Figure 1.
A total of 114 women with TNBC were enrolled in this study. All diagnostic, therapeutic, and follow-up procedures were conducted in one center, the Holycross Cancer Centre in Kielce, Poland, in the years 2013–2020. The mean age of women was 45.7 years (ranging from 27 to 75), the most common type of cancer was non-specific type, and in all patients, subcutaneous mastectomy was performed. The sentinel lymph node biopsy procedure was applied in 82 patients, in 61 cases after the neoadjuvant chemotherapy (NAC), and in 21 patients before the adjuvant chemotherapy (AC).
The mean number of sentinel lymph nodes was 3.13 (ranging from 1 to 9). The most common regimen of chemotherapy was 4 cycles of doxorubicin and cyclophosphamide followed by 12 cycles of paclitaxel – applied in 91 (80%) patients. Adjuvant chemotherapy was received by 23 patients, and in 30 patients it was applied as an adjuvant with capecitabine after neoadjuvant chemotherapy due to the lack of complete regression of cancer. Radiotherapy was applied in 43 out of 114 patients. In 38% of patients, a genetic mutation was established. We did not ask for the approval of the Ethics Committee due to the retrospective nature of the analysis. Upon admission to hospital, the written consent of the patients to be included in the study was obtained. Detailed patient characteristics and the types of treatment are depicted in Table 1.
Table 1
Basic characteristics of 114 triple-negative breast cancer patients
Statistical analysis
Basic statistics are presented as the mean ± standard deviation, median (Q1–Q3), the range or number and proportion, depending on the type of variables studied. Disease-free survival and overall survival were estimated by the Kaplan-Meier method. Survival time in months was calculated from the date of the treatment initiation to the date of the last follow-up, cancer relapse and/or death from any cause. The results of the analysis are presented as 1-, 3-, and 5-year survival probabilities with a 95% confidence interval (95% CI). The relationships between selected clinicopathological features and life status were tested with the c2 test. The influence of selected prognostic factors on the risk of death was analyzed using the Cox proportional hazards models. Univariate and multivariate analyses were interpreted using regression coeffcients and hazard ratios with their respective 95% CIs. P values < 0.05 were considered statistically significant. All statistical analyses were performed using R (version 3.6.3).
Results
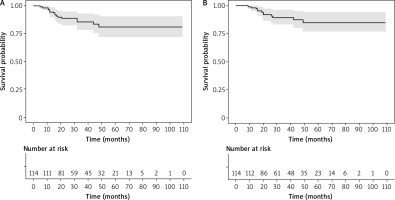
In the study group of patients (n = 114), the probability of disease-free survival at 1, 3, and 5 years was 0.945 (0.903, 0.989), 0.854 (0.784, 0.930), and 0.808 (0.721, 0.906), respectively. The probability of overall survival at 1, 3, and 5 years was 0.982 (0.958, 1.000), 0.894 (0.832, 0.959), and 0.850 (0.770, 0.939), respectively (Fig. 2). The median survival time based on the longest survival time observed in the data was 8.1 years. There were 12 deaths and 16 cancer relapses in the analyzed group. The most common cause of death was dissemination of the disease to the liver, bones, and the brain. In all of the deceased, dissemination occurred within 3 years after the diagnosis. In 2 patients, locoregional recurrence occurred in the skin and regional lymph nodes. Four patients are alive with cancer dissemination to the bones and lymph nodes of the mediastinum after the salvage systemic treatment. A significant relationship with life status was observed for clinical stage, use of sentinel lymph node biopsy, use of radiotherapy, and complete pathological regression after neoadjuvant chemotherapy (Table 2). In stages I and II, death occurred in 7% of patients, and in stage III in 36%. In the group with SLNB, death was reported in 6%, and after axillary lymphadenectomy in 20% of patients. Death in the group of patients treated with adjuvant radiotherapy was reported in 25%, and in 1.4% without this form of treatment. Pathological complete regression (pCR) of the cancer after NAC was observed in 52 patients. In this group, death occurred in 4% of patients. In the group without complete cancer regression, death was reported in 16% of patients.
Table 2
Relationships between vital status and selected clinicopathological characteristics
Clinicopathological features were included in the univariate Cox proportional hazard models, the significance of which was p ≤ 0.2 in the previous analysis (Table 2). Negative values of regression coefficients indicated relatively better, positive, and relatively worse prognoses in the case of the studied category of the analyzed feature compared to the reference category. Based on univariate models, a significant association with the risk of death was observed for the clinical stage, the type of surgery, the presence of SLNB, and radiotherapy (Table 3). All features presented in Table 3 were qualified for further multivariate analyses. Based on the multivariate Cox model, a significant relationship with the risk of death was observed for gene mutations, the type of surgery, radiotherapy, and the presence of complete pathological regression (Table 4). The presence of a genetic mutation, complete cancer regression in the pathological examination, and the absence of indications for radiotherapy significantly reduced the risk of death, and skin-sparing mastectomy compared to nipple-sparing mastectomy increased the risk of death. Clinicopathological features (except for types of surgery) were included in further multivariate analyses, the significance of which was p ≤ 0.2. The reason for the exclusion of types of surgery from the further analysis was an uneven distribution of the frequency of the examined features, which affected the reliability of the results, visible in the example of a highly wide and non-diagnostic 95% CI.
Table 3
Univariate Cox proportional hazard model based on selected clinico-pathological characteristics of patients with triplenegative breast cancer
Table 4
The eight-factor Cox proportional hazard model for the triple-negative breast cancer patients
Based on the 5-factor (final) Cox model, all included features (except for age) had a significant impact on the risk of death (Table 5). In conclusion, the presence of a genetic mutation, adjuvant chemotherapy, complete pathological regression, and the absence of indications for postoperative radiotherapy were associated with better prognosis. In the analyzed model, age as a key factor taken into account in all time-to-event analyses did not show a significant relationship with the risk of death.
Table 5
Final Cox proportional hazard model for the triple-negative breast cancer patients
Discussion
Triple-negative breast cancer is a highly aggressive form of malignancy. TNBC is associated with both a higher and an earlier risk for relapse. Hazard rates for distant recurrence are the highest for TNBC within the first 3 years following the diagnosis, relapses after 5 years are uncommon, and death is associated with dissemination, as was confirmed in our analysis. The results of treatment in our group are positive. Many women, particularly those with a low stage of cancer, achieved a complete response after chemotherapy, avoided aggressive surgical treatment, and did not need radiotherapy in addition to surgery.
Systemic treatment
In the analyzed time in our hospital, only chemotherapy was used in relation to the systemic therapy. No monoclonal antibodies or poly-ADP ribose polymerase (PARP) inhibitors which are presently accessible were used [10]. Invariably, an anthracycline- and taxane-based regimen remains the standard of care for TNBC patients. In our group, we used preoperative systemic treatment in 80% of women, but 20% of patients, in whom the cancer was detected in stages IA and IIA (with the tumor less than 3 cm), received chemotherapy after surgical treatment, and the decision of the multidisciplinary team was the surgery upfront. Neoadjuvant chemotherapy allows for the reduction of the volume of the primary tumor and the regional nodes, which can facilitate more options for surgical treatment. Nowadays, preoperative systemic treatment is most commonly applied, even in patients with early-stage disease. However, according to the NSABP B-27 trial, where preoperative or postoperative doxorubicin and cyclophosphamide with docetaxel were compared, there was no significant difference in disease-free survival (DFS) or overall survival (OS) [11]. In our study TNBC patients had a worse survival with NAC than with AC. This fact may be related to the low stage of cancer at diagnosis. These findings contrast with those of NSABP B-18, EORTC 10902, and the IBBGS, three large randomized trials evaluating NAC vs. AC. Those trials did not find a difference in survival between breast cancer patients receiving NAC and patients receiving AC. However, there was no selection according to the clinical subtype of breast cancer in those studies. Other prior studies had shown conflicting results in outcomes specifically for TNBC patients treated with NAC vs. AC [12–14]. Knowing that triple-negative breast cancer is significantly sensitive to chemotherapy, we can expect complete pathological regression in many patients. Although pCR patients have better outcomes than non-pCR patients, a number of those with pCR still have disease recurrence and breast cancer-related deaths. Lastly, this study confirms the previously described increasing trend in the use of NAC in TNBC patients [15]. In our group 83% of patients reached pCR and only 4% of them died, in comparison to 16% in whom pCR was not achieved. Since the year 2018, we have been using capecitabine due to the lack of complete regression of the cancer (30 women in our group). Five patients died. In all of them, the cancer was detected in clinical stage III.
Surgical treatment
The choice of surgery remains one of the most difficult issues in the treatment of TNBC [16]. The general trend in breast cancer, including TNBC, is the desire not only for oncological radicalism but also to ensure a good cosmetic result, which is possible with organ-preserving or reconstructive plastic surgery. It should be noted that the choice of method of surgical intervention depends on the decisions of the surgeon and the patient. Study results indicate that women with TNBC who undergo breast-conserving therapy do not have a worse prognosis than those who undergo mastectomy [17, 18]. According to Rippy, 27% of patients refused to undergo organ-preserving surgery in favor of mastectomy, but the patients’ choice depends on their understanding of this aspect of treatment [19]. Subcutaneous mastectomy is currently increasingly used as a method of surgical treatment of patients with breast cancer. Psychological aspects are also important in determining eligibility for this method of treatment, and many patients choose this form of therapy also because of the fear of radiotherapy, which is an inseparable part of conserving treatment. However, in patients with triple-negative breast cancer, in whom BRCA mutations are more common than in other biological types of breast cancer, mastectomy is the recommended method, and patients are rarely treated with breast preservation. The patients from the analyzed group were treated in the years 2013–2020. During that period we observed in our institution an upward trend for subcutaneous mastectomies. The decision to perform a mastectomy was made by the doctor and the patient. During this period the number of subcutaneous mastectomies with immediate reconstruction increased from 2% to 25% of all surgical procedures for women with breast cancer. The proportion of breast-conserving treatment at that time was about 50–60%. We also observed more sentinel lymph node biopsies than axillary lymph node dissections. At present, SLNB is a standard for patients with clinically negative lymph nodes, regardless of the biological subtype of the breast cancer. In the analyzed group, skin-sparing mastectomy was used in only 2 women. The decision to perform a mastectomy with removal of the nipple-areola complex was dictated by the higher stage of the disease and the proximity of the tumor to the skin of the areola and nipple; the distance was several millimeters, and therefore the decision was made to remove the nipple-areola complex for radical treatment. In the univariate analysis, SSM was a prognostic factor but it was not confirmed in the multivariate analysis. The use of axillary lymph node dissection was associated with a worse prognosis. The reason for this was the stage of the cancer and the necessity of more aggressive local treatment. The cancer in all women who died was detected in stages II and III with clinically positive lymph nodes. In 7 of them, axillary lymph node dissection was performed. There has also been a general trend in recent years for the rejection of extensive lymph node dissections in favor of sentinel lymph node biopsy. The condition of the axillary lymph nodes is one of the most important prognostic factors. Currently, the sentinel lymph node biopsy, in many cases, has replaced axillary lymphadenectomy and is also performed in patients after systemic treatment. In the group of 61 patients, a sentinel node biopsy was performed after systemic treatment. Only in one patient, in 1 sentinel lymph node, was cancer metastasis detected. A complete pathological response after neoadjuvant chemotherapy is a positive prognostic factor and, additionally, axillary lymphadenectomy can be avoided. In our study, the majority of patients underwent sentinel node biopsy, with a significant reduction in the toxicity of this surgical treatment. In 52 out of 91 women treated with neoadjuvant chemotherapy, complete pathological regression was achieved, which allowed most patients to undergo the SLNB procedure. Several clinical trials have found that complete axillary lymph node dissection had no significant effect on the overall survival, but reduced the risk of locoregional recurrence [20–22]. Axillary recurrences are very rare, which appears to be the rationale for not commonly using axillary dissection [23, 24].
BRCA status
Triple-negative breast cancer widely harbors BRCA gene mutations, more commonly than in other biological types of breast cancer. For patients with a diagnosed BRCA mutation, primary chemotherapy followed by surgery seems to be the most optimal therapy option. Research has shown that the mutation is a factor contributing to the complete pathological regression of cancer [7, 25]. In patients with triple-negative breast cancer and BRCA mutation, mastectomy is the recommended method, and patients are rarely treated with breast preservation [7]. In a systematic review, it was found that breast-conserving surgery was associated with a greater rate of ipsilateral breast cancer recurrence in BRCA mutation carriers. However, it was not associated with adverse short- and long-term survival outcomes. Breast-conserving surgery should be offered as an option to BRCA mutation carriers with proper preoperative counseling [26]. In the analyzed group, 20 patients achieved pCR and only 2 out of 43 patients with mutation died.
Radiation therapy
Radiotherapy is a valuable treatment method for breast cancer patients. It is particularly important after breast-conserving treatment, but when used in patients after mastectomy, it has a positive effect on local control and overall survival. Indications for radiotherapy occur in patients after mastectomy mainly due to metastases to regional lymph nodes, and the positive role of radiotherapy in TNBC patients has been proved [27–29]. In our group, radiotherapy was used in patients with a high primary stage of cancer. For patients with early-stage disease, there were no indications for irradiation. Forty-three patients were irradiated because of metastases to regional lymph nodes – 10 after adjuvant chemotherapy, and 33 women after mastectomy. Out of 12 patients in our group who died, 11 were postoperatively irradiated, but only in 1 case did we note locoregional (skin of the thorax) recurrence. What is interesting, in patients in whom mastectomy was the first stage of the treatment, radiotherapy of regional lymph nodes replaced axillary dissection in 9 cases. Patients in whom 1 or 2 sentinel nodes were positive are alive after radiotherapy of regional lymph nodes.
Conclusions
The results of treatment with subcutaneous mastectomy with immediate reconstruction are positive. The early stage of the cancer is associated with a better prognosis. The effective systemic therapy can be used before as well as after the surgery. Complete pathological regression after systemic treatment, particularly in patients with BRCA1 mutation, is a good prognostic factor and can help diminish the range of surgery in the axilla region. In particular, the sentinel node biopsy can be used in most patients even after neoadjuvant chemotherapy. In early cases, regional lymph node irradiation can replace axillary lymph node dissection. However, it is necessary to remember that in women with an early stage of breast cancer, usually we should propose breast-conserving therapy. Nevertheless, very often, despite the early stage of the cancer, women, for various reasons, wish to undergo subcutaneous mastectomy with reconstruction.