Synchronous and/or metachronous primary triple lung cancer is an extremely rare pathology [1]. Unilateral localisation in a single lung appears to be exceptional.
According to the literature, the prevalence of synchronous primary triple lung cancer is low, ranging from 0.3% to 4.6% [2].
Triple primary lung cancer is a real diagnostic challenge for the physician. It is generally confused with intrapulmonary metastases and tumour recurrence, which can now be distinguished thanks to the frequent use of increasingly powerful computed tomography (CT) scanners, as well as other examinations such as FDG18 positron emission tomography (PET-SCAN), bronchoscopy, bronchial echo-endoscopy, and fluorescence endoscopy [3, 4].
Moreover, only anatomopathological examination can provide a definitive diagnosis, leading to staging of the tumour lesion, which determines the therapeutic approach to be adopted.
We report two cases of unilateral synchronous triple primary lung cancer.
A 58-year-old man with a history of chronic obstructive pulmonary disease, a chronic smoker (40 packs/year), admitted for 3 months of progressive dyspnoea associated with dry cough with minimal haemoptysis, and weight loss of less than 6 kg of his initial weight of 75 kg.
The physical examination was normal, and a thoraco-abdominopelvic CT scan revealed a pulmonary mass of the right inferior lobe measuring 35 × 38 mm, extending into the parietal pleura and without involvment of adjacent chest wall structures, associated with right pulmonary hilar and subcarinal lymph nodes. A transparietal biopsy was performed for anatomopathological analysis, leading to the diagnosis of a well- to moderately differentiated adenocarcinoma infiltrating the lung parenchyma.
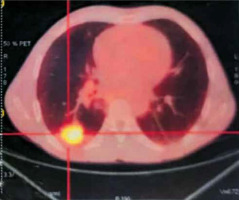
Magnetic resonance imaging (MRI) of the brain was normal, and 18 FDG positron emission tomography (PET-SCAN) revealed an active tumour mass in the posterobasal segment of the lower lobe of the right lung (SUVmax = 7.2), associated with 2 micronodular lesions in the upper segment of the right upper lobe (SUVmax = 3.1), and pathological hypermetabolic lymph nodes in the right mediastinum (Figure 1). At the end of these examinations, the tumour was classified as T2N2M0 (stage IIIa) and neoadjuvant chemotherapy was initiated, consisting of 4 sessions of treatment, the evolution of which was marked by stability of the lesion on the follow-up thoraco-abdominopelvic CT scan, thus indicating surgery, after a normal operability assessment (spirometry with FEV1: 83.2%, cardiac echography, biological work-up). A right lower lobectomy was performed, with lymph node dissection of the Barety lodge and subcarinal site, wedge-type resection of the nodular processes of the right upper lobe, and pleural biopsy via posterolateral thoracotomy. Anatomopathological analysis of the surgical specimen showed a predominantly acinar pulmonary adenocarcinoma with an associated lepidic component in the lower lobe, and a moderately differentiated squamous cell carcinoma associated with high-grade dysplastic lesions in the upper lobe, with no lymph node or pleural tumour infiltration.
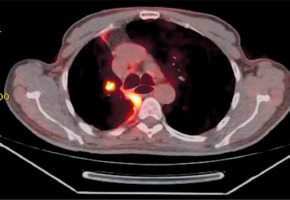
Finally, 4 months later, the patient developed a nodule in the right upper lobe on the follow-up PET CT scan (Figure 2), which was biopsied transthoracically and analysed as scleronodular Hodgkin lymphoma. Given this diagnosis, the patient received 3 courses of chemotherapy, without regression of the tumour lesion, necessitating readaptation of the chemotherapy protocol. It was at the fourth session that the patient died from severe sepsis.
A 64-year-old man, type 2 diabetic, with high blood pressure, a surgical history of laryngectomy plus definitive tracheotomy following squamous cell carcinoma of the larynx in 2020, a chronic smoker (35 packs/year), who had quit smoking in 2018.
Two years later, as part of a follow-up for his squamous cell carcinoma of the larynx, a cervico-thoracoabdominal CT scan revealed a right Fowler’s nodule suspected of malignancy. A scanno-guided biopsy with histological analysis was in favour of a moderately differentiated squamous cell carcinoma of questionable primary or metastatic origin.
Magnetic resonance imaging (MRI) was normal, and an 18 FDG PET scan showed hypermetabolism of the right Fowler’s nodule (SUVmax = 4.7) with no fixation elsewhere, indicating surgery.
A right lower lobectomy plus lymph node dissection of Barety’s lodge by single-port thoracoscopy (U-VATS) was performed, with pT1bN0M0.
In 2023, the patient was readmitted to the department because of the discovery, as part of carcinological follow-up, of a subpleural nodule in the dorsal segment of the right upper lobe (18 × 11 mm) and a nodule in the apical segment of the right upper lobe (11 × 7 mm), with a small right pleural effusion (Figure 3), on cervico-thoracoabdominal CT scan.
The physical examination was normal, but a PET-scan scan revealed hypermetabolism of the nodule in the dorsal segment of the right upper lobe and a ventral nodule in the middle lobe associated with a small, non-fixing pleural effusion.
After a normal preoperative workup, the patient underwent wedge resection by posterolateral thoracotomy for both nodules, then the operative specimen was sent for anatomical-pathological examination, the result of which was in favour of a well-differentiated infiltrating and keratinising squamous cell carcinoma (Figure 4 A) for the right upper lobe nodule, with a composite small-cell neuroendocrine carcinoma representing 60% of proliferation (Figure 4 C) and a glandular contingent representing 40% of proliferation for the middle lobe (Figure 4 B).
Figure 4
Pathological views in 40× magnification: A – squamous carcinoma, B – neuroendocrine carcinoma, C – adenocarcinoma

With this treatment, the patient progressed successfully, is in good health, and leads a normal life, remaining able to carry out his daily activities.
Triple primary lung cancer may be synchronous (occurring simultaneously) or metachronous (occurring at different times) [5]. According to the classification criteria for synchronous multiple primary lung cancer, described by Martini and Malamed [2], it is a secondary tumour, which is physically distinct and separate, or a secondary tumour that has a different histology from the primary tumour. If it has the same histology, it must be located in different segments or lobes. Metachronous multiple primary lung cancer, on the other hand, includes having a secondary cancer, which is histologically different, or identical to the first cancer, occurring at least 2 tumour-free years after the first tumour, and located in a different lobe, with absence of extra-pulmonary metastases at the time of diagnosis [6]. Our 2 clinical cases meet the criteria for synchronous multiple primary lung cancer.
Triple primary lung cancer is a real diagnostic challenge because it determines the patient’s therapeutic approach and prognosis. Its differential diagnosis with extra-pulmonary (lymphatic) or intrapulmonary (hematogenous) metastases and/or tumour recurrence is difficult. However, according to the literature, its distinction from a metastasis is facilitated by the use of rich and varied complementary examinations such as computed tomography, FDG18 positron emission tomography (PET-SCAN), bronchoscopy (through bronchoalveolar lavage and biopsy), bronchial echo-endoscopy, and fluorescence endoscopy, which allow the detection of lesions suspected of malignancy [3, 4]. In our 2 clinical cases, lesions suspected of malignancy were detected using computed tomography with FDG18 positron emission tomography. However, we believe that these 2 methods are insufficient, because some lesions may go unnoticed, as illustrated by the case report by Kashif et al. [3], in which bronchoalveolar lavage via bronchoscopy and accidental biopsy of a left endo-bronchial lesion played a major role in the diagnosis of 3 synchronous primary lung cancers on pathological examination.
In our first patient, however, only the suspicious lesion in the right lower lobe was biopsied transparietally for histological analysis, which came back suggestive of a well- to moderately differentiated adenocarcinoma classified as stage IIIa (T2N2M0). This diagnostic strategy differs from that of Yoon et al. [7], who, after detecting three suspicious lesions on CT scan, performed a simultaneous scanno-guided biopsy of all 3 lesions, resulting in the diagnosis of 3 histologically different primary lung cancers on pathological examination, with 3 different stages for each primary cancer. This would explain why chemotherapy was not beneficial for our patient, because after surgery, histological analysis of the surgical specimen was in favour of 2 synchronous primary cancers of different histological nature (adenocarcinoma for the right lower lobe, and squamous cell carcinoma for the isolated upper lobe nodule); thus, there were 2 different TNM stagings, calling into question the initial staging. Four months later, this patient presented with a right upper lobe nodule, suspected of malignancy on follow-up CT scan, biopsied and histologically analysed as primary Hodgkin lymphoma of the lung.
Nevertheless, it is vital to highlight that a pulmonary localisation of Hodgkin’s lymphoma is highly exceptional, accounting for 3% to 4% of extra-ganglionic malignant lymphomas and less than 1% of all lung cancers [8–12]. Diagnostic criteria are based on anatomopathological findings, limitation of the disease to the lung with or without minimal hilar lymph node involvement, and exclusion of any other localisation [8, 10, 11]. This was the case in our clinical observation, where the scleronodular form was diagnosed, which accounts for 60–70% of primary pulmonary Hodgkin’s lymphoma [8, 9]. Treatment is based on a combination of chemotherapy and radiotherapy, and it results in complete and prolonged remission after 5 years in 80-95% of localised forms [13]. In our case, only chemotherapy was initiated, which could explain why the tumour persisted despite chemotherapy sessions, making the patient vulnerable to any type of infectious aggression.
Similarly, in the case of our second patient, the cervico-thoracoabdominal CT scan and PET scan detected the suspected malignant lesion in the right lower lobe, and the scanno-guided biopsy with histological analysis was in favour of a moderately differentiated squamous cell carcinoma; the same result was obtained after surgery, when the histological analysis of the surgical specimen was conducted. One year later, as part of an oncological follow-up, a PET-SCAN was carried out, again suspecting 2 nodules (upper right lobe and middle lobe), based on which, after the operability (spirometry, cardiac echography, and biological work-up) and resectability assessments came back normal, a wedge resection by posterolateral thoracotomy was performed. Anatomopathological examination of the surgical specimen identified a well-differentiated infiltrating and keratinising squamous cell carcinoma (right upper lobe nodule) and a small-cell neuroendocrine carcinoma (ventral middle lobe nodule), concluding in a synchronous triple primary lung cancer, just like the first case, as it met the criteria for synchronous multiple primary lung cancer according to Martini and Melamed [2].
Finally, another rare feature of our case report is that our 3 synchronous primary lung cancers were unilateral, on the right lung, which differs from the case reports of the following authors: Kashif et al. [3], Zardo et al. [4] and Yoon et al. [7], in which the 3 synchronous primary lung cancers were distributed over both lungs and were thereby bilateral.
In conclusion, synchronous primary triple lung cancer is a rare form of lung cancer, which is difficult to differentiate from metastatic pathology or tumour recurrence. Similarly, localisation to a single lung seems exceptional, with a rich and varied histological type, as rare as primary Hodgkin’s lymphoma of the lung, to name but one.
Similarly, while CT scans and FDG18 positron emission tomography are effective in detecting lesions suspected of malignancy, they are limited in detecting endo-bronchial lesions, which are easier to detect with bronchoscopy, bronchial echo-endoscopy, and fluorescence endoscopy. Anatomopathological examination alone establishes the diagnosis and then leads to staging of the tumour lesion, guiding the right therapeutic attitude and determining the patient’s prognosis.