Spermatic cord sarcomas comprise a rare genitourinary malignancy that presents a challenging diagnostic and therapeutic pathway. They represent less than 5% of all soft-tissue sarcomas and less than 2% of malignant urologic tumours. The most commonly reported subtypes are liposarcoma, leiomyosarcoma, and rhabdomyosarcoma, with other rare variants including undifferentiated pleomorphic sarcoma and desmoplastic round cell sarcoma [1–3]. Due to the rarity of the condition’s nature, it is difficult to evaluate large patient cohorts. However, existing literature of up to 22 patients have indicated the existence of a highly diverse histological field. Between the different histological subtypes, undifferentiated pleiotropic sarcoma is the rareest subtype with reported incidence as low as 4.5% of all spermatic cord and scrotal sarcomas [4]. The spermatic cord is by far the commonest site of sarcoma occurrence within the genitourinary tract, accounting for approximately 30% to 90% of all cases, depending on the reporting institution. Epidemiologically, the incidence of spermatic duct sarcoma is bimodal, with patients aged 16–20 years showing a high incidence rate for rhabdomyosarcoma, and patients older than 60 years constituting the second peak of incidence, with liposarcoma and leiomyosarcoma being the most common subtypes [4]. In most cases, a person with a paratesticular tumour will experience a swelling on one side of the groin (inguinal) or a mass in the scrotum. This swelling or mass may or may not be painful and sometimes there is also a buildup of fluid around the testicle (hydrocele) [2–4]. In rare cases, the first sign of the condition might be sudden severe scrotal pain due to tissue death (necrosis) or bleeding within the tumour. Because the symptoms can be vague, it is crucial to accurately diagnose a paratesticular tumour before surgery to distinguish it from other benign conditions in the groin or scrotum. These benign conditions include inguinal hernias, hydroceles, lipomas, haematoceles, tuberculosis of the epididymis or orchitis (inflammation of the testicle and epididymis), and testicular cancer [4, 5]. Making an accurate diagnosis is essential to avoid incomplete removal of the tumour or contamination of the surgical area during surgery. It must be noted, however, that despite advances in imaging accuracy and new protocols for soft tissue imaging, several cases of spermatic duct sarcomas cannot be differentiated from soft tissue contents of an inguinoscrotal hernia. The diagnosis of spermatic duct sarcoma is often complex due to the rarity of the condition and the absence of well-defined preoperative diagnostic criteria [4–6]. Ultrasound imaging is a common diagnostic tool that can suggest the presence of a tumour, showing a hypoechoic extra testicular scrotal mass that is highly vascularised on Doppler imaging. In the presence of a scrotal mass, ultrasonography serves as the initial imaging modality for localisation purposes. It effectively differentiates intratesticular from paratesticular lesions but may have limitations in distinguishing a herniated adipose tissue component from a lipomatous mass. For a more comprehensive evaluation of disease extent beyond the inguinal canal, computed tomography (CT) and magnetic resonance imaging (MRI) are valuable tools [5–7]. These modalities provide detailed information regarding the dimensions and topography of the mass, and its relationship with surrounding anatomical structures. Definitive diagnosis of paratesticular tumours hinges upon histopathological examination of the mass tissue. This can be achieved through surgical resection or percutaneous core biopsy. Due to the relative rarity of these tumours, there is a paucity of robust clinical evidence to guide the development of multimodality treatment strategies. Within this case report, we present the extremely rare case of a 66-year-old man who presented with an inguinal mass that proved to be an undifferentiated pleiotropic spermatic duct sarcoma. To the best of our knowledge, this is one of the few reported undifferentiated spermatic duct sarcomas along with the course of treatment. Within the scope of this report, we also aim to briefly present the current knowledge on diagnosis, and current trends on the management of a rare soft tissue tumour, which can be unexpectedly encountered by the general surgeon.
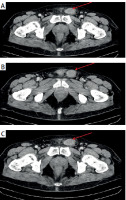
A 66-year-old male patient, previously fit and well, presented within the surgical assessment unit of the General University Hospital of Alexandroupoli complaining of a growing left inguinal mass, symptomatic for approximately 4 weeks. The patient reported gradual increase in the mass size and brief bouts of inguinal pain, especially when standing up. Upon physical examination, a palpable mass of maximum diameter of approximately 4–5 cm was noted in the left inguinal canal, non-tender, well defined, soft, mobile, most resembling an indirect inguinal hernia. Due to the palpable soft tissue mass, which posed the clinical dilemma of intra-inguinal mass, the patient was further worked up with an abdominal computed tomography. The scan reported a heterogenous, soft tissue mass 4 × 3.6 cm in maximal diameter appearing to be stemming from the left spermatic duct, resembling a spermatic duct sarcoma. No other intra-abdominal abnormalities were noted, no enlarged regional or retroperitoneal lymph nodes seen, and no suspicions of local tissue infiltration were noted from the CT imaging (Figures 1 A–C). Laboratory parameters were all within the normal range. Testicular tumour markers, including β-HCG, lactate dehydrogenase (LDH), and α-fetoprotein, were also within the normal range. Based on the probable diagnosis of a spermatic duct sarcoma, the management was decided to be primarily surgical, with identification of the mass and complete excision with clear margins from the left spermatic duct. The patient was taken to the theatre as an elective case, with the possibility of an orchiectomy priorly discussed with the patient, as part of the necessary radical excision. The surgical approach was through an elliptical incision. The spermatic duct was delivered, and the spermatic duct dissected free. The left testicle was also delivered through the inguinal incision, with the sarcoma extending up to 6 cm from its distal border to the testis. The sarcoma was carefully excised free from the spermatic duct, with no intraoperative injury, and macroscopically free surgical margins. The specimen, along with surrounding fatty tissue, was sent to the histopathology lab for further investigation. Microscopically, the specimen exhibited signs of neoplastic tissue in collagenous background, with cellular polymorphisms, inflammatory cellular infiltrations, and histiocytes. Immunohistochemistry staining was positive for tumour markers CD99, CD68, a1-antithrypsin, vimentin, a-antichymothrypsin, Factor XIIIa, and CD10, as well as for a-SMA. MIB-1 was positive in approximately 3–5% of the cells. The above findings are in line with undifferentiated pleiotropic spermatic duct sarcoma. Fibrous/fatty surrounding tissue was negative for associated lymph nodes. The patient recovered well from the operation and was able to return home the same day. No postoperative complications were noted in the early postoperative period. The patient was discharged and referred to the oncology department for further management. The patient was followed-up after 6 months with abdominal and chest CT scans, that did not indicate any signs of local recurrence, local or distal lymphadenopathy, or distal metastasis. Further management was continued by the oncology team for possible adjuvant chemoradiation therapy after radical surgery. Spermatic cord sarcomas, despite their rarity, exhibit a highly aggressive clinical course. Radical surgical resection remains the cornerstone of treatment, potentially followed by adjuvant therapy based on disease stage [8, 9]. However, a significant challenge arises due to the frequent occurrence of misdiagnosis and the potential omission of radical orchiectomy from the preoperative discussion. This can lead to a higher risk of incomplete tumour removal.
Figure 1
A–C – Different computed tomography (CT) images noting the mass (red arrow) attached to the spermatic duct seen laterally
The management of these malignancies is further complicated by the paucity of high-quality data and established treatment guidelines in the literature. Notably, in this study, only 3 patients with positive surgical margins lacked preoperative imaging, while 2 had CT scans alone, and 7 had a combination of ultrasound (US), CT, and magnetic resonance imaging (MRI) [10]. These findings suggest potential limitations in current imaging techniques or shortcomings in surgical planning. Additionally, 5 patients received non-radical interventions at other institutions, with 4 requiring subsequent resections for disease recurrence or persistence, highlighting a potential pattern. This variability in primary tumour management likely further increases the complexity of subsequent adjuvant and salvage treatments, ultimately leading to more challenging clinical scenarios [7–10]. Extensive research has been conducted to identify factors influencing recurrence and survival rates in genitourinary (GU) sarcomas. Russo et al. analysed 1583 sarcomas, reporting that smaller tumour size (< 5 cm), lower histological grade, and complete surgical resection were favourable prognostic indicators for survival. However, it is noteworthy that this cohort included only 14 paratesticular sarcomas [11]. Dotan et al. presented a larger cohort with extended follow-up, ultimately identifying 57 sarcomas of paratesticular origin. Their multivariate analysis revealed that tumour size and the absence of metastasis at diagnosis were the sole significant predictors of disease-specific survival [10]. Furthermore, Stojadinovic et al. demonstrated that positive surgical margins significantly increased the risk of local recurrence (28% vs. 15%, p < 0.001), alongside a heightened risk of distant metastases and disease-related mortality [12]. Wang et al. investigated predictors associated with long-term survival in a cohort of 188 adult patients with GU sarcomas. Their multivariate analysis identified patient age less than 50 years and incomplete surgical resection as independent predictors of both recurrence-free and overall survival [13]. The primary treatment for spermatic duct sarcoma is surgical, with the aim of complete tumour removal. Given the high frequency of locoregional recurrence, wide radical resection is crucial, and negative surgical margins should be aimed for [11–14]. While chemotherapy regimens and radiation treatment remain viable adjuncts, surgical treatment with complete tumour excision (as stated by negative surgical margins) remains the gold standard for the treatment. According to the literature, a significant difference is seen in mid-term survival rates for patients with adequate tumour excision when compared to excision with positive surgical margins, which further supports surgical treatment as the most effective treatment option [15, 16]. Adjuvant radiotherapy can be proposed, especially in cases where surgical margins are positive or in higher-grade tumours [12]. Similarly to other genitourinary sarcomas, the tumours studied exhibited a concerning tendency to recur and metastasise [11–13]. A significant portion (65%) of the patients experienced recurrence in the original location, with an average time of 25 months before reappearance. Additionally, 65% of patients developed metastasis, with an average of 40 months before detection. Several factors probably contribute to these high rates. One potential explanation is patient hesitancy to seek medical attention. Delays in diagnosis and limited access to healthcare, especially in developing countries, could also play a significant role. The prognosis for spermatic duct sarcoma varies depending on several factors, including the histological subtype, grade of the tumour, and the success of surgical intervention. One study reported a 5-year cancer-specific survival rate of 91.3%, indicating a relatively good prognosis for patients who undergo successful treatment [8–10]. However, all patients who died of the disease had positive surgical margins, underscoring the importance of achieving negative margins during surgery. Spermatic duct sarcoma is a rare but serious condition that requires careful diagnostic and therapeutic management. The key to improving outcomes lies in early detection, accurate diagnosis, and complete surgical removal of the tumour.
Within this report, we present the case of a patient who sought early surgical consultation and therefore was able to undergo radical surgery with no further treatment needed. Although lacking in guidelines, current strategies are centred around negative surgical margins. Ongoing research and clinical trials are essential to better understand this disease and develop more effective treatments.










