INTRODUCTION
Aseptic meningitis is defined as an acute developing meningitis in which the pathogen cannot be detected in the Gram staining of the cerebrospinal fluid (CSF) culture and a parameningeal focus cannot be determined [1]. Malignancies, autoimmune diseases, viral and fungal infections, as well as aseptic meningitis due to certain drugs are frequently seen. Antibiotics, intravenous immunoglobulin (IVIG), non-steroidal anti-inflammatory drugs are often considered responsible. The incidence following IVIG infusion is 0.067%; however, symptoms may start to show up anywhere from 6 h to 4 days following infusion. It is challenging to diagnose and is easily overlooked because of symptoms and clinical findings that might arise from a variety of conditions, including headaches, stiff necks, and vomiting [2, 3]. The causes underlying aseptic meningitis following IVIG remain unknown [4].
We report a patient with Noonan syndrome (NS) who was diagnosed with common variable immunodeficiency (CVID) due to recurrent illness and started on IVIG replacement therapy. After the first IVIG infusion, the patient developed aseptic meningitis, which has never been documented in the literature before.
CASE REPORT
A 17-year-old male patient with NS was admitted to the hospital with a preliminary diagnosis of meningitis/encephalitis with complaints of severe headache, neck stiffness, photophobia and subfebrile fever that developed in the last few days.
The patient was conscious, in good general condition, and had normal vital signs, and central imaging and fundus examinations were normal. Laboratory values are shown in Table 1. No cells were seen in the CSF cell count, CSF microprotein was 75.4 mg/dl, CSF glucose was 58 mg/dl (120 mg/dl in the blood). There was no growth in the PCR panel and CSF culture of large meningitis including viral and bacterial agents. The onset of the patient’s symptoms within 2 days after IVIG intake, the inability to grow the viral and bacterial agents in cultures, and laboratory findings indicated that the patient had aseptic meningitis due to IVIG. During the follow-up of the patient, his complaints regressed, and he was discharged on the seventh day of hospitalization. When the patient received IVIG afterwards, the product brand and IgG concentration were altered. It was advised, therefore, to take prior premedication (methylprednisolone 1 mg/kg, pheniramine maleate 1 mg/kg, and paracetamol 10 mg/kg). He experienced sporadic headaches rarely during the follow-up after receiving IVIG infusions, but he fared well on anti-inflammatory medication. He complained of headaches in recent months and began receiving a subcutaneous immunoglobulin medication.
TABLE 1
Laboratory results of the patient
Our patient was also followed up by the pediatric endocrinology department with the diagnosis of NS due to hypothyroidism and growth retardation. He applied to the pediatric allergy-immunology outpatient clinic with the complaint of urticaria plaques that had been going on for 3 months and appeared almost every day. While investigating the etiology of chronic urticaria, we learned that the patient had been sick frequently since infancy and often had mild respiratory infections. The patient had suffered from bronchitis three times in the past year and had been hospitalized three times due to high fever. His parents were not related and there was no known immunodeficiency in the family.
No cause was found in the examinations of the patient for chronic urticaria. The results of routine hematological, biochemical and hormonal tests were normal. The patient’s repeated immunoglobulin values were as follows – IgG: 462/438 mg/dl, IgA: 42/44.9 mg/dl and IgM: 27/42 mg/dl. Anti-HBs: negative, anti-CMV IgG: negative, anti-rubella IgG: low positive (42 IU/ml), anti-A: 1/64 and anti-B: 1/64. Flow cytometric lymphocyte subpopulation analysis showed normal T, B, NK cell distribution. Based on this anamnesis, clinical symptoms/signs and hypogammaglobulinemic laboratory results, the patient was diagnosed with CVID and 0.5 g/kg IVIG replacement therapy was started once a month. The first IVIG was given to the patient at a concentration of 10% at 0.5 g/kg in 4 h and no reaction was observed during the infusion. The patient’s complaints about recurrent respiratory tract infections decreased after IVIG and the patient achieved significant clinical benefit. Interestingly, urticarial lesions did not recur after IVIG treatment was initiated. Furthermore, Sanger DNA sequence analysis showed a heterozygous mutation of p.Met504Val (c.1510 A>G) in the PTPN11 (protein tyrosine phosphatase non-receptor type 11) gene. This mutation has previously been identified in the HGMD (Human Gene Mutation Database) for NS (#CM013423). (Written informed consent was obtained from the patient and his parents for the patient to be presented.)
DISCUSSION
Noonan syndrome is a genetic disorder with characteristic facial appearance, short stature, and congenital heart diseases, mostly autosomal dominant inherited syndrome. Although it is seen with a frequency of 1/1,000–2,500 in the population, its actual frequency is not known exactly due to its mild phenotypes. Pulmonary stenosis, hypertrophic cardiomyopathy, nutritional issues, delayed motor development, visual and hearing difficulties, hepatosplenomegaly, bleeding disorders, behavioral and emotional abnormalities may coexist with this representation in individuals due to its clinical and genetic variability [5]. Although it is thought to be associated with advanced paternal age, approximately 50% of them have a pathogenic variant of PTPN11 [5–7]. This typical mutation has been detected in our patient.
Guidelines have been established to monitor the development of patients with NS and to take precautions for complications that may arise [8]. Some disorders that can be seen in the central nervous system as the cause of headache in NS are craniosynostosis, Arnold Chiari malformation, hydrocephaly, and Moyamoya disease. There is no screening or warning for immunodeficiency, even if the headings in the manual guidelines include cardiac examination, pre-anesthesia evaluation, growth. In the literature, immunodeficiency in NS is rarely defined [9, 10]. However, our patient was diagnosed with CVID by the pediatric allergy and immunology department due to recurrent lower respiratory tract infections, and was diagnosed with aseptic meningitis when he was followed up due to headaches that developed after IVIG treatment.
There are no data on the incidence of NS and post-IVIG aseptic meningitis, because it has not been reported in the literature previously. Aseptic meningitis is a rare complication of IVIG treatment and, although the frequency was noted as the highest in a 1994 study of 11%, the literature indicates an overall incidence of 0 to 1% [11], which is 0.067 per infusion when calculated for patients receiving recurrent infusions [3, 12].
Although immunoglobulin therapy was first used in the treatment of primary immunodeficiency disorders, it is now administered intravenously or subcutaneously as a replacement therapy, immunomodulator therapy (Kawasaki, Guillain-Barré syndrome, etc.), in autoimmune diseases, neuro-immunological and neuromuscular diseases. As the area of use has become widespread, the literature knowledge about its effects and side effects, and the characteristics of the application, has increased rapidly [13, 14].
The most common side effects after IVIG administration are shown in Table 2. The evidence suggests that immunoglobulin is responsible for the etiology of aseptic meningitis and that there is a temporary relationship with immunoglobulin therapy, that meningitis symptoms have improved continuously, and that 10% of the 71 patients in the literature have had recurrent patterns after administration of immunoglobulin [15].
TABLE 2
Almost half (36/71) of the cases of acute meningitis associated with IVIG reported in the literature were under the age of 18 years. It has been associated with underlying immune thrombocytopenia in children (17/36). The rate of IVIG administration, the variety of preparations and the CSF content were examined in detail in the cases. In most of the cases (2/3), pleocytosis was detected in favor of neutrophilia [16]. Contrary to what is generally known, our patient is under the age of 18, has NS and has been diagnosed with CVID, and there was no pleocytosis in his CSF.
The IgG level remains in the CSF for up to 48 h after termination of IVIG administration and decreases in concentration as the neurological symptoms of aseptic meningitis improve. It shows that IgG not only penetrates the meninges, but also enters the brain parenchyma and affects it [17].
The level of monocyte chemotactic protein-1 (MCP-1) was found to be significantly higher in the CSF of patients diagnosed with IVIG-induced meningitis. An increase in MCP-1 in the CSF may cause monocyte activation and aseptic meningitis development [4]. The second mechanism is the occurrence of meningeal stimulation, most likely after type III and type IV hypersensitivity to the drug. In addition, it is associated with acute leptomeningeal irritation and inflammatory cytokine release as a result of IgG-mediated complement activation or interaction between IgG and antigenic determinants in meningeal vessels [11] when serum IgG crosses the blood-brain barrier and is 4 times higher [3, 11].
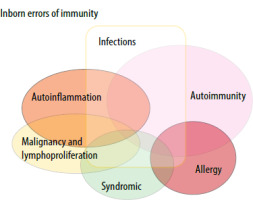
Primary immunodeficiency diseases are sometimes described in rare multisystemic genetic conditions. It is linked to innate immune defects, autoimmunity, allergies, syndromes, and infections (Figure 1). There are no clear data in the literature on the frequency of NS and immunodeficiency [18]. Syndromic primary immunodeficiencies are examined. The syndromes under this heading are classified by the International Union of Immunological Societies. Although NS is not well known under this heading, it has rarely been reported in some studies. To the best of our knowledge, no cases of aseptic meningitis have been previously identified in these syndromic genetic disorders [3].
FIGURE 1
Relationship of syndromic conditions with innate immune disorders and different diseases [9]
Measures to prevent the development of aseptic meningitis due to IVIG are controversial.
Although the first recommendation is not to repeat IVIG infusion, this situation can be interpreted differently according to the diseases. If reinfusion is necessary, the infusion should be slowed and spread out over days, or the use of other commercial products should be considered. Adequate fluid intake before infusion, pain relievers such as paracetamol (codeine may be added), anti-inflammatory drugs such as NSAIDs, antihistamines may be used [12]. Since it is more common in patients with a history of migraine, migraine prophylaxis is also recommended [11]. Corticosteroids are not considered beneficial in aseptic meningitis caused by IVIG [12]. In our patient, the recurrence of aseptic meningitis and headaches was prevented by switching/changing the IVIG product and the concentration and even the administration of the product intravenously to the subcutaneous route.
CONCLUSIONS
In immunodeficient patients presenting with signs of meningitis, IVIG intake should be questioned in the anamnesis, and aseptic meningitis should be among the differential diagnoses. In patients with aseptic meningitis, it seems rational to change the product and apply premedication in other IVIG administrations. Although the reason for this association is unknown, NS may also be a risk factor for IVIG-associated aseptic meningitis. However, more studies are needed to confirm this hypothesis.







