The emergence of acute mitral valve insufficiency (MI) due to papillary muscle rupture poses a clinical challenge demanding swift intervention. This report describes the intricate case of a 67-year-old man with profound MI and anterior-lateral papillary muscle avulsion. Our aim is to elucidate the intricate diagnostic path, surgical complexities encountered, and diverse postoperative challenges within this atypical clinical presentation. The patient’s symptomatology, characterized by angina, exertional dyspnea, and a history of vigorous physical activity, extended beyond routine manifestations of coronary artery disease, necessitating an in-depth diagnostic exploration.
Despite exhaustive efforts, the precise etiology of the papillary muscle rupture remained elusive, with ischemic factors conclusively ruled out. Notably, physical exertion emerges as the leading contender contributing to this complex pathology. There are few descriptions in the literature of nonischemic acute MI. Complete anterior papillary muscle rupture (PMR) caused by localized papillary muscle infarction was described by Sugawara in a case of angiographically normal coronary arteries. Pathologic examination showed both old fibrosis and new ischemic lesions in the same resected papillary muscle, suggesting that repeated localized subendocardial infarction caused spontaneous PMR [1]. Rato et al. described catastrophic antiphospholipid syndrome (CAPS), a rare and serious phenomenon requiring prompt recognition and treatment, presenting the case of a puerperal woman with systemic lupus erythematosus (SLE) [2].
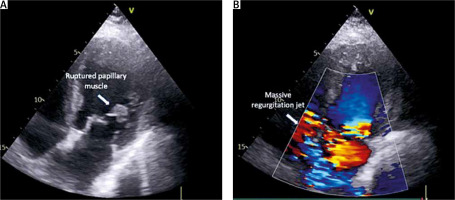
Upon remote consultation, the patient’s critical condition prompted a recommendation for urgent cardiothoracic surgery. Preoperative echocardiography revealed a pre-existing rupture of the papillary muscle, exacerbating the severity of mitral regurgitation, evident in a massive regurgitant flow (Figure 1). This necessitated an emergency mitral valve replacement using a 27 mm bioprosthesis (Edwards Magna, USA) and simultaneous pleural drainage due to substantial effusion. Mitral valve replacement (MVR) with a 27 mm bioprosthesis (Edwards Magna, USA) was deemed more suitable to address the extensive damage and ensure a more effective resolution of mitral regurgitation. This decision was influenced by the severity and complexity of the pathology, as well as the need for swift and decisive intervention to stabilize the patient’s critical condition. In summary, the choice of MVR over repair was driven by the observed extensive damage, intraoperative challenges, and the necessity for a robust solution to address the complexity of the patient’s cardiac condition. Immediate challenges, including the need for re-exploration due to postoperative bleeding and persistent pleural effusion requiring drainage after 10 days, underscore the intricate and multifaceted nature of managing cases of this complexity.
Figure 1
A, B – Echocardiographic image depicting a ruptured papillary muscle and Doppler echocardiogram illustrating a massive regurgitant jet through the mitral valve
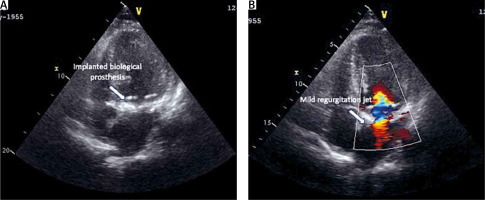
During the postoperative echocardiographic examination, the imaging revealed the successful placement of the mitral prosthesis in its anatomically correct position (Figure 2). However, a notable observation was the presence of a massive regurgitant flow, indicative of a potential detachment of the papillary muscle. Further investigation confirmed the postoperative complication of an isolated papillary muscle detachment, adding a layer of complexity to the patient’s recovery. The dynamic and evolving postoperative course demanded careful attention and a comprehensive approach to address diverse challenges that could arise. The implementation of anti-inflammatory therapy played a pivotal role in the postoperative care strategy, further complicating the nuanced clinical narrative. The elusive etiology of the papillary muscle rupture, combined with the significant pleural effusion, necessitated a proactive approach to address potential inflammatory factors contributing to complications, such as papillary muscle detachment. The implementation of anti-inflammatory therapy became imperative in navigating through this intricate clinical scenario, aiming to optimize healing, mitigate inflammation-related risks, and enhance the overall success of the surgical intervention. Following a 24-day hospitalization, the patient was discharged and transferred to a regional hospital for rehabilitation and extended care. Satisfactory recovery and subsequent home discharge after 31 days signify a successful conclusion achieved in managing the intricate challenges posed by the patient’s complex cardiac condition.
Figure 2
A, B – Surgically implanted mitral bioprosthesis in an echocardiographic examination, illustrating precise placement within the anatomical structure, and echocardiographic representation revealing a mild regurgitant flow through the mitral bioprosthesis, providing insight into the valve’s post-procedural performance
The central theme of the discussion revolves around the diagnostic complexities stemming from the elusive etiology of papillary muscle rupture. The exclusion of ischemic causes directs attention to physical exertion as the most plausible contributing factor to the described pathology. Fiore et al. proposed that the papillary muscle works as a shock absorber compensating for geometric changes of the left ventricular wall, linking the etiology of papillary muscle rupture to physical and mechanical strains on the papillary muscle [3]. Sultan et al. described mitral valve surgery for acute papillary muscle rupture, emphasizing expeditious operative intervention associated with acceptable operative and longer-term outcomes [4]. Gouda et al. described spontaneous papillary muscle rupture (PMR) as a rare cardiovascular emergency and highlighted the importance of rapid identification due to advances in imaging modalities and improved surgical management, leading to optimal outcomes in patients with spontaneous PMR [5]. This reframes the diagnostic and therapeutic approach, emphasizing the need for a meticulous exploration of patient history. The complex interplay between a multidisciplinary approach, timely surgical intervention, and vigilant postoperative care highlights the intricate management required in acute mitral valve insufficiency secondary to papillary muscle rupture.
In conclusion, this nuanced clinical scenario not only contributes to the evolving field of cardiovascular medicine but also serves as a testament to the resilience of diagnostic and therapeutic paradigms. The report underscores the need for thorough diagnostic exploration, collaborative healthcare efforts, and the continuous evolution of strategies to manage complex cardiovascular pathologies, especially in scenarios where a clear etiology remains elusive.






