Introduction
Overweight and obesity among pediatric patients are now becoming a major public health challenge worldwide. Excess body fat is associated with an increased likelihood of cardiometabolic diseases, lipid disorders, hyperglycemia and hepatic steatosis [1, 2]. Liver steatosis can have different etiologies [3, 4]. However, it is worth noting that hepatic steatosis in the course of obesity and related metabolic disorders is an important factor in the development of chronic liver disease. Considering the impact of metabolic disorders on the onset of hepatic steatosis, in 2023 an expert group proposed the introduction of the new term “metabolic dysfunction associated steatotic liver disease” (MASLD) [5]. The diagnosis of MASLD is associated with the presence of one of 5 cardiometabolic risk factors (excess body weight, hyperglycemia, abnormal blood pressure values, hypertriglyceridemia or reduced high-density lipoprotein cholesterol (HDL-C) levels) and liver steatosis detected by imaging studies or biopsy. Given the prevalence of metabolic disorders in children and adolescents, easily accessible markers are still being sought to assess the probability of their occurrence [6, 7].
Uric acid (UA) is the end product of purine metabolism, derived mainly from endogenous synthesis and only a small portion from exogenous sources. Currently, there is an increase in the prevalence of hyperuricemia in countries with Western lifestyle [8]. A role for UA in the pathogenesis of hypertension, insulin resistance (IR), type 2 diabetes, chronic kidney disease and metabolic syndrome (MetS) in adults has also been postulated [9]. The studies have observed higher serum UA values in children with liver steatosis compared to children without liver pathology [10, 11]. Based on studies in adults with hepatic steatosis, it has been observed that UA may be an additional criterion for metabolic disorders in patients with MASLD [12].
Therefore, the purpose of this study was to evaluate serum concentrations of UA in obese children and to determine the association of this parameter with MASLD and MetS in pediatric patients.
Material and methods
The controlled clinical study included consecutive overweight or obese children and adolescents admitted to our department with suspected liver disease. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). According to the World Health Organization (WHO), overweight was defined as BMI standard deviation (SD) score > 1, and obesity as BMI > 2 SD [13]. There is a lack of universal calculators of waist circumference (WC) percentile values in relation to age and sex in children and adolescents. Based on a recently published article [14], all children in the study group had WC ≥ 90th percentile. Blood pressure (BP) was measured at least three times at rest using an electronic sphygmomanometer and the average value was taken. Elevated BP was defined according to criteria for MetS [15]. In all participants, other liver diseases associated with steatosis were excluded, such as viral hepatitis, selected metabolic liver diseases (Wilson’s disease, α1-antitrypsin deficiency), cystic fibrosis, celiac disease, autoimmune hepatitis and toxic conditions. Exclusion criteria also included acute infection, taking drugs that affect blood pressure, lipid or carbohydrate metabolism, or alcohol consumption.
In all patients routine biochemical measurements were performed, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), total cholesterol, HDL-C and low-density lipoprotein (LDL-C) cholesterol, triglycerides (TG), UA, glucose, and insulin. Homeostasis model assessment of insulin resistance – HOMA-IR = (fasting insulin (mIU/l) × fasting glucose (mmol/l))/22.5) – was calculated to estimate IR. All assays were conducted according to the manufacturer’s instructions. The liver steatosis was diagnosed in abdominal ultrasonography performed in all participants. Carotid ultrasonography examinations were performed to evaluate carotid intima-media thickness (IMT) by the same experienced radiologist.
Patients were then divided into two groups of MASLD and non-MASLD based on laboratory and imaging findings according to the latest guidelines [5]. Patients included in the study were also assessed for the presence of MetS according to the International Diabetes Federation (IDF) criteria for children and adolescents. According to the criteria, MetS can be diagnosed at the age of 10 years and older; children aged < 10 years were excluded from the MetS analysis (n = 12) [15].
Data analysis was performed using the Statistica 12 software. Quantitative data were shown as median and quartiles (Q1-Q3). Qualitative variables were presented as absolute frequency and percentage. Statistical analysis was performed using the Mann-Whitney test for quantitative data and the chi-square (χ2) test for categorical variables. The relationship between serum UA levels and variables was analyzed by the Spearman rank-correlation test for nonparametric data. The receiver operating characteristics (ROC) analysis was performed to calculate the power of UA for detecting MASLD and MetS in overweight and obese children. The statistical significance level was set at p < 0.05.
The study was approved by the Bioethics Committee of the Medical University of Bialystok prior to patient recruitment, and the study is in accordance with the Declaration of Helsinki (approval number: R-I-002/427/2017).
Results
A total of 194 overweight/obese participants were recruited to the study, including 133 (68.56%) with MASLD. The characteristics of the groups with and without MASLD are shown in Table 1. There were significantly elevated levels of ALT, AST, GGT, total cholesterol, TG and UA in the MASLD group in comparison to the non-MASLD group. Also, the mean IMT was higher in children with MASLD than in those without liver steatosis.
Table 1
Characteristics of the study group divided according to the MASLD diagnosis
[i] MASLD – metabolic dysfunction associated steatotic liver disease, ALT – alanine aminotransferase, AST – aspartate aminotransferase, GGT – γ-glutamyltransferase, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, TG – triglycerides, HOMA-IR – homeostasis model assessment score, UA – uric acid, IMT – intima-media thickness, BMI – body mass index, SD – standard deviation, BP – blood pressure, NS – not significant
Table 2 shows the characteristics of the patients stratified by MetS presence. Considering the MetS components, parameters such as HDL-C, TG, glucose and the presence of abnormal blood pressure values were not analyzed. Patients with MetS were significantly older than those without MetS. Among the MetS group, hepatic steatosis was noted in more than 80% of children. Nevertheless, liver function tests such as ALT, AST, GGT were not statistically significantly different between the two groups. Higher insulin, HOMA-IR and UA values were observed among children diagnosed with MetS. In addition, the group of children diagnosed with MASLD and MetS (n = 40/49) had significantly higher uric acid levels than the group with MASLD without MetS criteria met (n = 88/133) (median values: 6.56 mg/dl vs. 5.71 mg/dl; p = 0.03) (data not shown).
Table 2
Analyzed parameters in metabolic syndrome (MetS) and non-MetS groups (n = 182)
[i] MetS – metabolic syndrome, ALT – alanine aminotransferase, AST – aspartate aminotransferase, GGT – γ-glutamyltransferase, LDL-C – low-density lipoprotein cholesterol, HOMA-IR – homeostasis model assessment score, UA – uric acid, IMT – intima-media thickness, BMI – body mass index, SD – standard deviation, NS – not significant
The following significant positive correlations were noted: UA with ALT (r = 0.29, p < 0.001), AST (r = 0.15, p = 0.04), GGT (r = 0.35, p < 0.001), TG (r = 0.15, p = 0.04), insulin (r = 0.20, p = 0.006), HOMA-IR (r = 0.22, p = 0.003), mean IMT (r = 0.25, p = 0.002) and BMI-SD (r = 0.19, p = 0.007). Only one negative correlation was observed between UA and HDL-C (r = –0.25, p < 0.001).
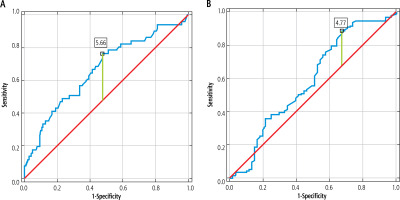
The ROC analysis presented in Table 3 and Figure 1 was performed to determine the predictive value of UA for distinguishing overweight/obese children with MASLD and MetS. A statistically significant result was obtained for UA in the MetS group (p < 0.001; AUC = 0.67 with 77% sensitivity and 52% specificity at the cut-off level 5.65 mg/dl). Borderline statistical significance was found for UA as a differentiating marker between MASLD and non-MASLD (p = 0.05).
Discussion
In our study, the prevalence of MASLD among overweight and obese children was 68%, while MetS was diagnosed in 26.92% of participants. Both children with MASLD and MetS had higher serum UA levels compared to children without these disorders. In addition, a number of correlations of UA with well-known cardiometabolic risk factors such as BMI-SD, TG, HDL-C, and IMT were observed. Interestingly, UA was helpful in differentiating between children with and without MetS, while only borderline statistical significance was observed for MASLD. Importantly, children with MASLD and MetS had higher UA values than children with liver steatosis without diagnosed MetS. Given our results, it is important to consider whether UA can be identified as another cardiometabolic risk factor in the diagnosis of MASLD.
The effect of hyperuricemia on hepatic steatosis remains unclear, but there are several hypotheses to explain the phenomenon. In recent decades, overconsumption of fructose from corn syrup and sugar-sweetened beverages has been observed. Excessive fructose intake stimulates the turnover of purine nucleotides, leading to the synthesis of UA from xanthine, which plays an important role in the onset and progression of fatty liver and the development of MetS [16]. Uric acid increases the production of reactive oxygen species and oxidative stress, which have pro-inflammatory effects. Hyperuricemia may also lead to fat accumulation in hepatocytes by inducing lipogenesis through the production of endoplasmic reticulum and activation of fatty acid synthase and acetyl-CoA carboxylase. Uric acid also affects endothelial function and nitric oxide bioavailability, causing IR [17]. IR plays a key role in the onset and progression of liver steatosis [18]. Although the purpose of our study was not to assess the effect of UA on the development of MASLD and MetS, we observed a positive correlation between UA and HOMA-IR, which also confirms the relationship between these parameters.
To date, one study evaluating the usefulness of UA in the diagnosis of MASLD in children has been published [19]. Di Sessa et al. reported a significant increase in the risk of diagnosis of hepatic steatosis in a group of obese children with hyperuricemia and MetS. This association was not observed in cases with absence of hyperuricemia and MetS criteria. In addition, children without MetS but with elevated UA had higher TG and blood pressure values, which are components of MetS. The coexistence of hyperuricemia and MetS was associated with a worse cardiometabolic profile in children. The inclusion of hyperuricemia as a diagnostic criterion for cardiometabolic risk was associated with an increase in MASLD diagnoses of about 15% [19]. Given the long-term consequences of the disease, close monitoring of a larger group of patients could have long-term benefits in terms of reduced morbidity and mortality.
Similarly to our observations, studies published among pediatric patients with fatty liver disease have shown elevated serum UA levels in patients with liver steatosis [10, 20, 21]. Kim et al. found higher UA values in children with nonalcoholic fatty liver disease (NAFLD) compared to obese peers without liver pathology. The authors also suggested the use of UA in combination with ALT, HOMA-IR, TG and GGT as a readily available risk marker of NAFLD in the pediatric population [21]. Moreover, several studies involving pediatric patients have observed that hyperuricemia was not only associated with a diagnosis of simple hepatic steatosis, but was also associated with an increased risk of nonalcoholic steatohepatitis (NASH) [10, 20]. In our study, UA levels were significantly higher among patients with MASLD, while their usefulness in differentiating MASLD in obese children reached only borderline statistical significance. The differences in our results can be explained by the different criteria for the diagnosis of MASLD and NAFLD. Not all studies observed an association between hyperuricemia and hepatic steatosis. Cardoso et al. noted that hyperuricemia was significantly associated with MetS, while it was not helpful in diagnosing NAFLD in obese children [22]. However, these data contradict the results obtained by Liu et al., who found that high UA values were more strongly associated with fatty liver disease than MetS [23]. Undoubtedly, more studies in children are needed to evaluate the utility of UA in the diagnosis of MASLD in children.
More research on the association between hyperuricemia and hepatic steatosis has been conducted in adults, but patients included in the studies were mainly classified as having NAFLD or metabolic associated fatty liver disease (MAFLD). A recently published study involving adults with hepatic steatosis suggested a potential role for UA as a criterion for cardiometabolic risk in the diagnosis of hepatic steatosis. During a mean follow-up period of 2.8 ±1.1 years, it was observed that in addition to standard cardiometabolic risk criteria (overweight, hypertension, hyperglycemia, high TG levels, low HDL-C levels), hyperuricemia increased the likelihood of NAFLD diagnosis [12]. Other studies have found that elevated UA levels were associated not only with MAFLD, but also with cardiovascular mortality [23, 24]. It seems interesting to investigate whether lowering UA levels would be associated with a reduction in the incidence of MASLD and its long-term consequences. Al-Shargi et al. observed that a reduction in UA levels in adult MASLD patients was associated with a reduction in hepatic steatosis measured by transient elastography [25]. It would be interesting to find out whether similar effects would be observed in children and whether this would have an impact on reducing cardiometabolic risk in pediatric patients.
The strength of our study is that we analyzed the associations between UA and multiple clinical and biochemical parameters in children with MASLD. Previously published studies have only described serum UA levels in children with MASLD without assessing parameters of liver function, lipid metabolism or IR. Our study provides more insight into the course of MASLD in pediatric patients. Studies conducted on children, due to the rarer presence of confounding factors and less advanced disease, may provide a more reliable model of disease development and progression than studies performed on adult patients. However, our work has several potential limitations. First, the number of patients was too small to draw definite conclusions. In addition, our study did not analyze participants’ dietary habits and physical activity, which could also have affected our results.
Conclusions
Our results suggest that UA may potentially be a readily available marker of metabolic dysfunction in children with MASLD. It seems important to determine UA in the serum of overweight/obese children to better monitor and observe the development of hepatic steatosis, which is considered a hepatic manifestation of MetS. There is a need for further studies in children to determine whether UA levels can be an indicator of MASLD onset and progression, and whether UA reduction can become one of the therapeutic options in this disease.