Introduction
Sarcoidosis is a chronic autoimmune granulomatous disease that may affect multiple organs and is characterized by the formation of noncaseating granulomas [1]. It is hypothesized that sarcoidosis occurs in genetically susceptible individuals and from interactions with environmental exposure [2]. The worldwide prevalence ranges from 1 to over 60 per 100,000, with a three-fold predominance among African Americans [3]. Because of the heterogeneity of organ involvement, the presentation is wide-ranging, from asymptomatic or non-specific complaints to classic organ-specific complaints.
Since hepatic sarcoidosis is scarce, evidence-based therapy recommendations have not yet been established. The incidence of hepatic sarcoidosis is underestimated due to its silent presentation [4], often discovered as part of a workup for elevated hepatic biochemical tests, with or without a background of systemic sarcoidosis, or while investigating radiologic lesions. Hepatic granulomas, a common finding, can have four histologic variants: noncaseating, caseating, fibrin ring, and lipogranulomas [5]. As most patients with hepatic granulomas are asymptomatic, no specific medical therapy is required; however, in patients with symptoms due to liver involvement or those at risk of liver complications, pharmacologic treatment may be considered. Glucocorticoids have been the mainstay of symptomatic sarcoidosis treatment for several decades; other possible medications include methotrexate, azathioprine, and infliximab [4].
Up to 95% of patients with clinically evident hepatic sarcoidosis are diagnosed by elevated alkaline phosphatase (ALP) and/or γ-glutamyl transferase (GGT) levels [4]. Ursodeoxycholic acid (UDCA) is used in the treatment for cholestatic liver disease, either as first-line treatment (primary biliary cholangitis) or off-label (primary sclerosing cholangitis); with hepatic sarcoid being a predominantly cholestatic liver disease, UDCA could be a first-line treatment. A few retrospective studies have demonstrated improvement in hepatic biochemical tests with the use of UDCA, but there are no prospective trials to date [6]. The effects of UDCA, prednisolone, or no treatment on liver-related symptoms and liver biochemistries in patients with hepatic sarcoid were analyzed in a retrospective cohort study. UDCA was associated with greater improvement in fatigue, pruritus, and liver aminotransferases compared to corticosteroids or no treatment [7]. A review recommends starting UDCA as the first-line treatment in primary hepatic sarcoid and only adding prednisone if UDCA does not achieve the intended symptomatic and/or biochemical improvements [8]. Due to the lack of prospective studies, we set out to evaluate the effects of UDCA on hepatic sarcoid in a single-center, open-label, prospective, pre-post study.
Methods
Key inclusion criteria were the diagnosis of systemic sarcoidosis with evidence of liver involvement as denoted by elevated liver-specific ALP with any of the following: granulomas on liver biopsy, hepatomegaly on imaging, clinically significant portal hypertension (via imaging or endoscopy) OR granulomas on liver biopsy (not attributable to infection) with concomitantly elevated liver-specific ALP. Key exclusion criteria were the presence of another liver disease (steatosis was allowed), currently being on UDCA, and prior intolerance to UDCA. Once enrolled, patients were observed for six months followed by six months of weight-based UDCA (13-15 mg/kg) divided into two daily doses. Patients had the option of a twelve-month UDCA extension for a total of up to 24 months of observation. There was an optional sub-study for this trial including the 13C-methacetin breath test (MBT or BreathID – courtesy of Meridian Biosciences). The primary endpoint was a decrease in ALP or GGT at month 6 of UDCA treatment. Key secondary endpoints included decreases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and in liver stiffness (kPa) as assessed by vibration-controlled transient elastography (VCTE), and changes in the MBT.
Results
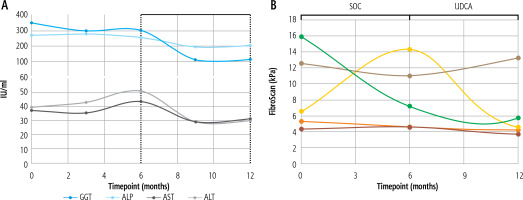
A total of 10 patients were screened from August 2018 to July 2020; seven met the criteria and were enrolled. The study was terminated prior to achieving target enrollment of 10 patients due to the difficulty in recruitment around the COVID-19 pandemic. Most patients were women (4/7; 57.1%) and African American (5/7; 71.4%). One patient dropped out during the first month of observation due to a new diagnosis of esophageal cancer. Six completed the 6-month observation and UDCA treatment periods. One patient stopped UDCA within the first month of active treatment due to the side effect of nausea. There was a decrease in ALP and GGT after six months of UDCA treatment compared to six months of observation (ALP – 257.6 to 202.2, p = 0.23; GGT – 302.5 to 111.8, p = 0.059; Fig. 1A), but this did not reach statistical significance (Fig. 1A). There were also decreases in all key secondary endpoints (ALT – 50.8 to 29.8, p = NS; AST – 40.3 to 31.2, p = NS, VCTE kPa – 8.3 to 6.3, p = NS) (Fig. 1A, B). Similarly to primary endpoints, none of the key secondary endpoints reached statistical significance. The results of the breath test were inconclusive due to the timing of patient visits during the COVID-19 pandemic, as most patients were unable to complete their on-treatment MBT.
Discussion
Single center enrollment and early termination resulting from recruitment challenges during the COVID-19 pandemic are the major limitations of our study. This study suggests a role for UDCA in the treatment of hepatic sarcoid, with improvements in hepatic biochemical tests and fibrosis as assessed through non-invasive tests. Though UDCA has been shown to decrease ALP and GGT in a variety of cholestatic liver diseases, its association with fibrosis regression has only been demonstrated in primary biliary cholangitis [9]. While one patient in the study reported nausea with UDCA (a known, yet uncommon side-effect), UDCA is generally well tolerated, easy to obtain, not immunosuppressive, and available in generic form, making it an ideal first-line agent for hepatic sarcoid. Unlike immunosuppressants, which tend to be cycled based on sarcoid disease activity, UDCA could be taken indefinitely without concern of immunosuppression adverse events, including increased risk for infection. In assessing a patient for treatment with UDCA, we suggest using it in those patients who have minimal extrahepatic sarcoid manifestations but persistently elevated liver-specific ALP and in those who are already on systemic treatment for sarcoidosis but continue to have elevated liver-specific ALP.