Summary
Bleeding complications after transcatheter aortic valve implantation (TAVI) are an important issue and negatively affect survival. The rate and impact of protamine sulfate (PS) administration on bleeding complications after TAVI remain unclear. In this analysis, PS administration did not decrease the primary endpoint (PE) rate. Female gender predicted PE occurrence. Randomized, placebo-controlled trials are required to accurately assess the impact of PS.
Introduction
Transcatheter aortic valve implantation (TAVI) is a rapidly emerging standard of care in patients with severe, symptomatic aortic stenosis, initially reserved for high surgical risk and inoperable patients, with the most recent studies showing a clear benefit in the low surgical risk cohort as well[1]. Numerous aspects of the pre-, peri- and post-procedural care have been studied and assessed in a wide array of randomized and non-randomized trials. However, reversal of unfractionated heparin (UFH) with protamine sulfate (PS) has never been thoroughly analyzed.
The current recommendation regarding reversal of UFH with PS is based on the expert consensus from 2012 [2], which recommends use of UFH in order to achieve activated clotting time (ACT) > 300 s as well as UFH reversal with PS in the case of TAVI via transapical access as well as transfemoral access with the exception of cases with minimal bleeding risk. Nonetheless, the clinical practice varies between centers – some use PS routinely [3], while others do so only in selected cases [4].
The actual impact of PS on reduction of bleeding complications is unknown. Furthermore, a pro-thromboembolic effect of PS cannot be excluded [5–7]. Both bleeding (major and life-threatening according to Valve Academic Research Consortium (VARC) criteria) [8] and thromboembolic complications increase mortality after TAVI [9], whereas minor bleeding complications may prolong the hospital stay. The occurrence of these complications in international TAVI registries in 30-day observation ranges from 9.7% in the case of major bleeding [10], 4.7% in the case of life-threatening bleeding [11] and 5% in the case of strokes [12]. There are no randomized studies assessing the impact of PS on frequency of bleeding and thromboembolic complications after TAVI, its side-effects and influence on mortality.
Aim
In order to appropriately plan a randomized controlled trial, we sought to first assess the frequency and impact of PS administration in this single-center retrospective analysis.
Material and methods
Study design and population
Two hundred and fifty-eight consecutive inoperable or high-risk patients with severe symptomatic aortic stenosis (aortic valve area (AVA) < 1.0 cm2 or indexed valve area less than 0.6 cm2/m2 or mean gradient > 40 mm Hg or maximum jet velocity > 4.0 m/s or velocity ratio < 0.25), who after the heart team decision underwent TAVI in an academic center between March 2010 and March 2016, were screened. The local ethics committee was informed about the study as per its guidelines for retrospective analyses.
All the procedures were performed in a hybrid operating room under general anesthesia (GA) or local anesthesia with conscious sedation. Balloon-, mechanically and self-expandable aortic valve prostheses of the first and second generation were used. Patients undergoing surgical cutdown as well as percutaneous access with a closure device were included. After obtaining the vascular access, all patients received a bolus of 5000 IU of UFH followed by additional boluses if necessary to achieve the target ACT of > 300 s. The administration of PS was at the operators’ discretion and the dosage was based on the amount of UFH administered as well as the ACT at the end of the procedure. In the case of antithrombotic treatment before and after TAVI, patients without indications for chronic oral anticoagulation (OAC) were given loading doses of 300 mg of aspirin and clopidogrel within 24 h before TAVI, and then continued 75 mg daily after the procedure. In patients requiring chronic OAC, the treatment was stopped 2–3 days before the procedure in order to obtain an international normalized ratio (INR) value < 2 in the case of vitamin K antagonists (VKA) and 1–2 days before the procedure depending on the renal function in the case of non-vitamin K antagonists. After TAVI oral anticoagulation was restarted as soon as deemed safe, with additional bridging with low-molecular weight heparin in patients receiving VKA.
The exclusion criteria were other-than-transfemoral access, conversion to aortic valve replacement, and procedural death defined as death during or immediately after the procedure.
Definitions and endpoints
Bleeding complications were defined according to Valve Academic Research Consortium 2 (VARC-2) criteria. Coronary artery disease was defined as a history of percutaneous coronary intervention (PCI), post coronary artery bypass grafting (CABG) status or presence of one or more lesions of the epicardial coronary vessels with > 70% diameter stenosis in arteries larger than 2 mm (> 50% for left main stem).
The primary endpoint (PE) of the study was a major bleeding complication according to VARC-2 at 48 h after the procedure. The secondary endpoints were the composite of major and minor bleeding complications according to VARC-2 at 48 h after the procedure and thromboembolic events (stroke, transient ischemic attack, myocardial infarction) within 48 h after the procedure.
Statistical analysis
Continuous variables, expressed as means ± SD, were compared between the PS and control groups using Student’s t-test or the Mann-Whitney U-test depending on the distribution pattern. The Shapiro-Wilk test was used to confirm or reject normal distribution of each continuous variable. Categorical variables, expressed as counts and percentages, were compared using the χ2 test or Fisher’s exact test, as appropriate. An univariate backwards likelihood ratio logistic regression model was used to identify predictors of the primary and secondary endpoints. Variables from the univariate analysis and baseline variables that were different between groups (with a p-value ≤ 0.10 difference) were included in the multivariate analysis. Results are presented as odds ratio (OR) with 95% confidence interval. All probability values reported are 2-sided and a value < 0.05 was considered to be significant. All data were processed using the SPSS software, version 22 (IBM SPSS Statistics, New York, US).
Results
Population
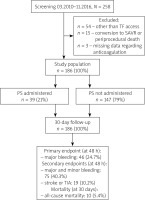
Of the 258 consecutive patients screened, 54 (21%) underwent TAVI via other-than-transfemoral access, 15 (5.8%) patients required conversion to surgical aortic valve replacement or died during or immediately after the procedure and in 3 (1.1%) definite data about periprocedural anticoagulation and PS use were missing. The study flow-chart is presented in Figure 1. The overall study population consisted of 186 patients. The mean age was 80.1 years, there were 96 (52%) females, almost 75% of patients had hypertension, 37% had diabetes and almost half (48.4%) were in New York Heart Association (NYHA) class III or IV. In over half (55.9%) of the procedures a vascular closure device was used (Prostar (Abbott Vascular Inc.) in 90% and Proglide (Abbott Vascular Inc.) in 10% of cases). Detailed baseline data are shown in Table I.
Table I
Baseline and procedural characteristics of the study population along with endpoint rates
[i] AR – aortic regurgitation, BMI – body mass index, BSA – body surface area, GFR – glomerular filtration rate, COPD – chronic obstructive pulmonary disease, LVEF – left ventricle ejection fraction, MR – mitral regurgitation, NYHA – New York Heart Association, PS – protamine sulfate, TIA – transient ischemic attack, UFH – unfractionated heparin.
Protamine sulfate administration
Thirty-nine (21%) patients received protamine sulfate at the end of the procedure in order to reverse the unfractionated heparin effect. The mean dose was 0.48 mg/100 IU of UFH. The group receiving PS had higher ejection fraction (56.3% vs. 50.1%, p = 0.02), received more UFH/kg (99.9 vs. 90.3 IU, p = 0.01) and more often underwent the procedure via percutaneous access with a closure device (82.1% vs. 49%, p ≤0.001, Table I).
Endpoints
The primary endpoint of major bleeding was observed in 46 patients (24.7% of the entire population; in the majority of the cases (n = 43) the bleeding was related to the access site). The composite endpoint of major and minor bleeding was observed in 75 patients (40.3% of the study cohort, 29 minor bleeding complications (15.6% of the entire group) – all related to the access site). There were 3 life-threatening bleeding complications (1.6%) and 54 patients required transfusion of blood products (29%). Thirteen strokes and seven transient ischemic attacks were observed (7% and 3%, respectively). The 30-day all-cause mortality was 5.4%.
In a multivariate analysis of the primary endpoint, only female gender (odds ratio (OR) = 2.2, confidence interval (CI) 1.08–4.4, p = 0.03) was identified as an independent predictor of PE occurrence (Table II). In a multivariate analysis of the secondary endpoint encompassing major and minor bleeding again female gender (OR = 2, CI: 1.06–3.84, p = 0.03) as well as GA (OR = 2.23, CI: 1.13–4.63, p = 0.02) and number of international units (IU) of UFH per kg (OR = 1.02, CI: 1–1.03, p = 0.02, per 1 IU/kg increment) were independently associated with the endpoint occurrence (Table III). Multivariate analysis of occurrence of ischemic events demonstrated a significant negative impact of stroke or TIA in the past (OR = 3.85, CI: 1.31–11.3, p = 0.01) and a protective effect of balloon- or mechanically expandable prosthesis use (OR = 0.25, CI: 0.07–0.92, p = 0.04). Protamine sulfate administration resulted in a numerically higher stroke or TIA rate (OR = 2.46, CI: 0.89–6.75, p = 0.08) (Table IV).
Table II
Uni- and multivariate logistic regression analysis of major bleeding occurrence
[i] AR – aortic regurgitation, BMI – body mass index, GFR – glomerular filtration rate, COPD – chronic obstructive pulmonary disease, CI – confidence interval, IU – international units, LVEF – left ventricle ejection fraction, MR – mitral regurgitation, NYHA – New York Heart Association, OR – odds ratio, TIA – transient ischemic attack, UFH – unfractionated heparin.
Table III
Uni- and multivariate logistic regression analysis of the composite of major and minor bleeding endpoint occurrence
[i] AR – aortic regurgitation, BMI – body mass index, GFR – glomerular filtration rate, COPD – chronic obstructive pulmonary disease, CI – confidence interval, IU – international units, LVEF – left ventricle ejection fraction, MR – mitral regurgitation, NYHA – New York Heart Association, OR – odds ratio, TIA – transient ischemic attack, UFH – unfractionated heparin.
Table IV
Uni- and multivariate logistic regression analysis of stroke or TIA occurrence
[i] AR – aortic regurgitation, BMI – body mass index, GFR – glomerular filtration rate, COPD – chronic obstructive pulmonary disease, CI – confidence interval, IU – international units, LVEF – left ventricle ejection fraction, MR – mitral regurgitation, NYHA – New York Heart Association, OR – odds ratio, TIA – transient ischemic attack, UFH – unfractionated heparin.
Protamine sulfate and endpoints
PS administration did not impact the occurrence of the remaining endpoints (Table V). More detailed subgroup analysis failed to demonstrate a protective effect of PS administration and PE occurrence in females (OR = 0.51, CI 0.2–1.31, p = 0.16), patients who received more than 92 IU of UFH per kg (mean UFH/kg value of the entire cohort, OR = 0.52, CI: 0.18–1.55, p = 0.24) and patients who underwent a procedure with a closure device (OR = 0.91, CI: 0.39–2.11, p = 0.8).
Table V
Impact of protamine sulfate administration on primary and secondary endpoints occurrence
Discussion
Impact of protamine sulfate on bleeding and thromboembolic complications
The main finding of our study is that PS administration during transfemoral TAVI did not decrease the rate of bleeding complications, irrespective of the approach – surgical cutdown or percutaneous access with closure device.
Since the initial studies assessing the feasibility and efficacy of TAVI, protamine sulfate administration has always been recommended [1] as a natural antidote to UFH with little or no consideration for the potential rebound anticoagulation due to PS short half-life (7 min as compared to heparin’s 60–90 min) as well as possible rebound thrombosis after sudden UFH reversal [13]. Despite the unpredictability of the PS effect as well as the well-proven negative impact of bleeding complications on survival [9], PS has never undergone scrutiny in the TAVI setting; therefore its true impact on bleeding complications remains uncertain.
Furthermore, a pro-thromboembolic effect of the PS cannot be excluded. In a randomized-controlled trial assessing UFH neutralization after a carotid endarterectomy, Fearn et al. concluded that reversing UFH with PS may predispose to thrombosis of the internal carotid artery and stroke [5]. In our study, a numerically higher stroke and TIA rate was observed in patients receiving PS. This along with absence of an impact on bleeding rates, should prompt caution and further investigation of protamine sulfate use in TAVI patients.
Protamine sulfate administration
Recommendations regarding UFH reversal with PS are based on an expert consensus from 2012 and advice to reverse UFH with PS in the case of transapical TAVIs as well as transfemoral ones with the exception of cases with minimal bleeding risk – they do not, however, define the minimal bleeding risk. In reality, centers around the world undertake an individual approach and some use PS routinely [3], while others do so only in selected cases [4].
In our analysis, 21% of the overall population received PS at the end of the procedure, while in the subgroup of patients treated via percutaneous access the percentage was 30.8%. The data regarding the exact rate of protamine administration are rarely reported and therefore difficult to compare. In a study assessing ACT-guided UFH administration, Bernelli et al. reported that 16.3% of patients have received PS [14]. The mean protamine sulfate dose in our study – 0.48 mg/100 IU UFH – seems plausible in light of UFH’s half-life time of 60–90 min and the ability of 1 mg of PS to neutralize 100 IU UFH[15]. Patients receiving PS more often underwent procedures with closure devices and received more unfractionated heparin per kg of body weight. The first finding is most likely a result of a more confident hemostasis achieved during a surgical cut-down approach. The tendency to administer PS in patients who have received a higher dose of anticoagulation is self-explanatory. The overall lower ejection fraction in patients not receiving PS may be due to the known negative impact of PS on blood pressure and hemodynamics [16].
Bleeding complications
The rate of major bleeding complications of 24.7% is higher than in randomized-controlled trials, but in line with real-life cohorts of TAVI patients [4, 13, 17] – in a meta-analysis of 3519 patients, the reported rate of bleeding complications according to VARC criteria ranged from 26.8% to 77% [18]. Multivariate logistic regression analysis identified female sex as an independent predictor of primary endpoint. A number of studies have shown an increase in bleeding and vascular complications among women undergoing percutaneous procedures in general [19–21]. A negative impact of female gender on bleeding complications after TAVI was previously demonstrated by the Paris–Rotterdam–Milano–Toulouse in Collaboration (PRAGMATIC) group – in their analysis of 986 patients, female sex doubled the risk of life-threatening and disabling bleeding events and increased the risk of vascular complications by 60% [22]. In a multivariate analysis of the composite of major and minor bleeding occurrence in addition to female gender, also GA and the number of IU of UFH per kg were identified as independent endpoint predictors. In the majority of studies comparing general anesthesia and conscious sedation there are no significant differences in terms of bleeding complication rate [23]. Although Gauthier et al. reported a significantly higher number of vascular complications, the difference in bleeding complications was only nominal [3]. Brecker et al. reported a similar rate of bleeding among 490 propensity-matched GA and conscious sedation TAVI patients [24]. In the so far unpublished SOLVE-TAVI randomized trial assessing anesthesia types, bleeding complications were not part of the primary or secondary endpoints [25]. The impact of body-weight-adjusted unfractionated heparin dose on bleeding has been shown in the past, including our own material [26]. In a previously mentioned study, non-ACT guided UFH administration resulted in significantly higher UFH doses and was associated with an almost six-fold increase in bleeding complication rates [13].
Limitations
The main limitation of our study is its relatively small sample size and retrospective character. Incorporating transfemoral procedures performed via surgical cutdown and percutaneously with device closure with the inherent differences between the two methods could have influenced the results. Study endpoints were collected retrospectively and not independently adjudicated. The decision-making process behind PS administration was not documented. The exclusion of periprocedural deaths precluded a meaningful analysis of the impact of bleeding complications on mortality.