Introduction
Uterine myoma is the most prevalent benign tumor in the female reproductive system, with an incidence of 20–25% [1]. Common clinical symptoms include monthly irregularities, miscarriage, pressure-related symptoms, and reproductive dysfunction [2–4]. While hysterectomy and myomectomy are traditional surgical treatments for uterine myoma, uterine artery embolization (UAE) has gained popularity since its introduction in 1995 as a minimally invasive and uterus-preserving nonsurgical option for those seeking to avoid surgery or preserve the uterus.
The UAE has been recognized as a beneficial therapeutic option for hysterectomy in several investigations, including multiple randomized controlled trials (RCTs) [4–8]. The RCTs evaluated myomectomy or hysterectomy with UAE and showed comparable outcomes in terms of quality of life at 1, 5, and 10 years of follow-up [4–8]. Hysterectomy may have a major impact on ovarian function due to the removal of the ovarian branch of the uterine artery during the procedure [9]. UAE may maintain ovarian function better than hysterectomy [6]. To date, few studies have examined the postoperative ovarian function in individuals with uterine myoma who underwent UAE versus hysterectomy.
Aim
To compare the postoperative ovarian function in individuals with uterine myoma who had UAE against hysterectomy.
Material and methods
Study selection
This meta-analysis was done as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria and has been registered at INPLASY.COM (No. INPLASY202420021).
A search was employed in Wanfang, Web of Science, and PubMed databases to identify relevant publications as of December 2023. The search terms used were: ((uterine fibroid) AND ((embolization) OR (UAE))) AND ((((surgery) OR (resection)) OR (myomectomy)) OR (hysterectomy)). The following inclusion criteria were applied: (a) studies comparing hysterectomy with UAE for uterine myoma; (b) ovarian function tests (pre- and postoperatively) were reported; and (c) the language used was not restricted. The exclusion criteria included: (a) research without human subjects; (b) sample sizes less than 20; and (c) continuous data that were not presented as mean ± standard deviation (SD).
Data extraction
Two authors separately retrieved pertinent data from the research, resolving discrepancies via discussion with a senior author. The extracted data consisted of the first author, publication year, country, study design, quality assessment scores, patient count, age, gender distribution, ovarian function tests (including pre- and postoperative FSH, LH, E2), and uterus and uterine myoma volume measurements (pre- and postoperatively).
Quality assessment
The quality of RCTs was assessed via the Cochrane risk-of-bias methodology, which categorized reporting, selection, detection, attrition, performance, and other biases as low, high, or uncertain risk.
The quality of observational studies was evaluated using the Newcastle-Ottawa Scale (NOS), which allocates points to each study according to outcome, comparability, and selection (3, 2, and 4 points, respectively) criteria. A NOS score of 7 or above indicated research of high quality.
Endpoints
This meta-analysis examined the preoperative and postoperative (at 6 months) volume of the uterus and uterine myoma, as well as the preoperative and postoperative (at 3, 6, and 12 months) FSH, LH, and E2 levels.
Statistical analysis
The meta-analysis and related analyses were performed using Stata v12.0 and RevMan v5.3. Continuous variables were compared via mean differences (MD) values along with 95% CIs. Heterogeneity was evaluated via the Q test and the I2 statistic (an I2 > 50% was suggestive of considerable heterogeneity). Random-effect models could be employed where heterogeneity was considerable, while fixed-effect models were utilized in other cases. Sensitivity analyses were performed via a “leave one out” approach to identify causes of heterogeneity. The presence of publication bias was assessed by Egger’s test, with a significance level set at p < 0.05.
Results
Study selection
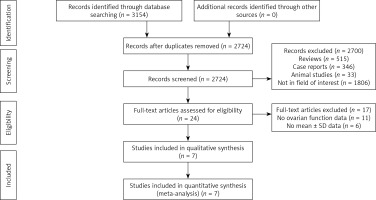
Initially, we identified 3154 articles using the search approach. After eliminating duplicate articles (n = 430), 2724 articles were evaluated. We removed 2700 unrelated papers and examined the remaining 24 acceptable publications. Seventeen papers were removed for not having ovarian function tests, and seven studies were included in our meta-analysis (Figure 1).
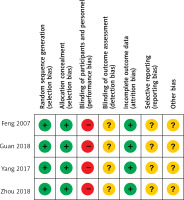
Out of the 7 investigations analyzed [10–17], four were RCTs [12, 13, 16, 17] while the other 3 were retrospective studies. The quality of the four RCTs is shown in Figure 2, while the NOS scores of the three retrospective investigations varied from 7 to 8 (Table I). The investigations were all carried out in China between 2007 and 2018.
Table I
Characteristics of included studies
| No. | First author | Publication year | Country | Study design | Number of patients | Age [years] | NOS | ||
|---|---|---|---|---|---|---|---|---|---|
| UAE | Hysterectomy | UAE | Hysterectomy | ||||||
| 1 | Cao [11] | 2018 | China | Retrospective | 80 | 80 | 45.9 ±7.0 | 8 | |
| 2 | Feng [12] | 2007 | China | RCT | 31 | 36 | 35.62 ±4.73 | 37.82 ±5.12 | – |
| 3 | Guan [13] | 2018 | China | RCT | 51 | 51 | 37.91 ±7.43 | 38.10 ±7.23 | – |
| 4 | Liu [14] | 2014 | China | Retrospective | 45 | 45 | 41–47 for all | 7 | |
| 5 | Luo [15] | 2014 | China | Retrospective | 66 | 40 | 32–45 for all | 7 | |
| 6 | Yang [16] | 2017 | China | RCT | 18 | 18 | 38.8 ±3 | 39.1 ±3.3 | – |
| 7 | Zhou [17] | 2018 | China | RCT | 43 | 43 | 40.6 ±2.3 | 41 ±2.4 | – |
A total of 334 patients who had undergone UAE and 313 patients who had undergone hysterectomy were included (Table II). Table II displays the pre- and postoperative (at 3, 6, and 12 months) values of FSH, LH, and E2.
Table II
Sex hormone level before and after treatments
| Studies | Groups | FSH [U/l] | LH [U/l] | E2 [pmol/l] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||||||
| 3 months | 6 months | 12 months | 3 months | 6 months | 12 months | 3 months | 6 months | 12 months | |||||
| Cao [11] | UAE | 8.33 ±1.4 | 9.98 ±1.28 | NG | NG | 34.31 ±5.02 | 33.84 ±4.93 | NG | NG | 664.2 ±104.8 | 839.5 ±167.8 | NG | NG |
| Hysterectomy | 8.25 ±1.34 | 9.07 ±1.34 | NG | NG | 33.01 ±5.44 | 24.16 ±6.29 | NG | NG | 679.2 ±120.4 | 289.5 ±66 | NG | NG | |
| Feng [12] | UAE | 6.92 ±1.44 | 7.49 ±1.68 | 7.15 ±2.08 | 7.08 ±1.89 | 9.64 ±2.43 | 9.95 ±1.95 | 10.56 ±2.1 | 10.12 ±2.19 | 58.49 ±4.77 | 56.31 ±4.34 | 56.67 ±5.36 | 56.15 ±6.89 |
| Hysterectomy | 6.97 ±1.61 | 8.93 ±2.71 | 9.1 ±3.47 | 9.98 ±1.55 | 9.94 ±1.67 | 10.99 ±1.24 | 14.02 ±2.91 | 15.13 ±1.18 | 57.13 ±4.75 | 52.3 ±4.45 | 50.38 ±7.86 | 47.98 ±4.89 | |
| Guan [13] | UAE | 6.74 ±1.37 | NG | 7.17 ±1.42 | 7.15 ±1.69 | 9.95 ±1.45 | NG | 10.64 ±1.53 | 10.27 ±1.62 | 56.72 ±6.34 | NG | 57.52 ±5.43 | 55.96 ±6.09 |
| Hysterectomy | 7.24 ±1.51 | NG | 9.62 ±1.73 | 10.37 ±1.77 | 10.47 ±1.27 | NG | 12.91 ±1.38 | 14.13 ±1.49 | 56.26 ±5.21 | NG | 53.08 ±5.02 | 49.19 ±4.15 | |
| Liu [14] | UAE | 13 ±5 | NG | 14 ±4 | 13 ±4 | 18 ±6 | NG | 18 ±7 | 18 ±6 | 286 ±48 | NG | 267 ±43 | 280 ±51 |
| Hysterectomy | 14 ±5 | NG | 15 ±4 | 15 ±4 | 17 ±6 | NG | 18 ±5 | 17 ±6 | 274 ±38 | NG | 247 ±30 | 274 ±36 | |
| Luo [15] | UAE | 6.98 ±1.23 | NG | 7.18 ±1.41 | 7.14 ±1.68 | 9.32 ±1.48 | NG | 10.63 ±1.52 | 10.26 ±1.63 | 56.71 ±4.44 | NG | 57.51 ±5.46 | 55.97 ±6.08 |
| Hysterectomy | 7.05 ±1.53 | NG | 9.63 ±1.72 | 10.37 ±1.76 | 9.29 ±1.31 | NG | 12.18 ±1.37 | 14.12 ±1.48 | 56.88 ±4.37 | NG | 53.07 ±5.01 | 49.18 ±4.16 | |
| Yang [16] | UAE | 6.95 ±1.5 | 7.46 ±1.6 | 7.2 ±2 | 7.02 ±1.22 | 7.5 ±1.71 | 9.8 ±1.69 | 6 ±2.03 | 8.7 ±2.05 | 157.1 ±4.69 | 178.95 ±4.71 | 157.16 ±5.29 | 194.92 ±5.55 |
| Hysterectomy | 5 ±1.49 | 9 ±2.47 | 19.1 ±3 | 19.89 ±1.18 | 7.4 ±1.6 | 11.1 ±1.59 | 14.02 ±3 | 15.2 ±1.19 | 158 ±4.87 | 90.26 ±4.29 | 51 ±7.02 | 47.59 ±5 | |
| Zhou [17] | UAE | 6.8 ±2.12 | 7.31 ±2.42 | NG | NG | 9.52 ±3.11 | 10.16 ±3.35 | NG | NG | 55.14 ±3.18 | 57.23 ±3.15 | NG | NG |
| Hysterectomy | 6.62 ±2.14 | 9.13 ±2.8 | NG | NG | 9.27 ±3.22 | 13.28 ±4.21 | NG | NG | 54.47 ±3.25 | 49.34 ±3.21 | NG | NG | |
Change of uterus volume
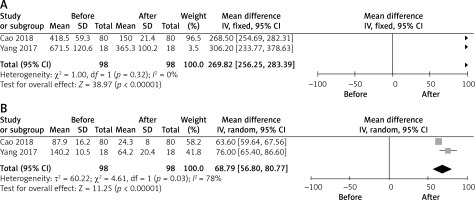
Two investigations documented the volume of the uterus both before and after undergoing UAE [11, 16]. The combined findings showed a substantial reduction in uterine volume after UAE treatment (MD = 269.82, 95% CI: 256.25–283.39, p < 0.00001, Figure 3 A). The heterogeneity was not statistically significant (I2 = 0%). Publication bias could not be evaluated due to the limited number of publications available for this specific outcome.
Change of uterine myoma volume
Two investigations documented the volume of uterine myoma before and after UAE [11, 16]. The combined findings showed a substantial reduction in uterine myoma volume after UAE treatment (MD = 68.79, 95% CI: 56.80–80.77, p < 0.00001, Figure 3 B). There was a substantial level of heterogeneity, with an I2 value of 78%. Unfortunately, sensitivity and publication bias analyses were not possible due to the limited number of papers available for this outcome (n = 2).
FSH
Preoperative FSH levels were recorded in all investigations, and the combined preoperative FSH levels were similar across the UAE and hysterectomy groups (MD = 0.12, 95% CI: –0.37–0.61, p = 0.63, Table III). There was a substantial level of heterogeneity, with an I2 value of 69%. The sensitivity analysis revealed that the research by Yang [16] was the cause of the heterogeneity. The pooled preoperative FSH levels were similar across the UAE and hysterectomy groups even after excluding Yang’s investigations (p = 0.46). The Egger’s test showed a minimal probability of publication bias with a p-value of 0.118.
Table III
Pooled results of pre- and postoperative FSH
Four studies examined the FSH levels 3 months after surgery [11, 12, 16, 17]. The combined FSH levels at 3 months post-surgery were similar across the UAE and hysterectomy groups (MD = –0.92, 95% CI: –2.59–0.75, p = 0.28, Table III). There was a substantial level of heterogeneity (I2 = 92%). The sensitivity analysis identified the research by Cao and He [11] as the cause of the heterogeneity. The 3-month postoperative FSH level was considerably lower in the UAE group compared to the hysterectomy group after excluding Cao’s data (p < 0.00001). The Egger’s test revealed a minimal probability of publication bias (p = 0.176).
Five studies examined the FSH levels 6 months after surgery [12–16]. The combined FSH level at 6 months post-surgery was notably lower in the UAE group compared to the hysterectomy group (MD = –3.89, 95% CI: –6.32––-1.46, p = 0.002, Table III). There was a substantial level of variability (I2 = 97%). The sensitivity analysis revealed that the research by Yang [16] was the cause of the heterogeneity. The 6-month postoperative FSH level remained considerably lower in the UAE group compared to the hysterectomy group after excluding Yang’s data (p < 0.00001). The Egger’s test showed a modest probability of publication bias (p = 0.15).
Five studies examined the FSH levels 12 months after surgery. The combined FSH level at 12 months post-surgery was notably lower in the UAE group compared to the hysterectomy group (MD = –4.86, 95% CI: –8.87–-0.85, p = 0.02, Table III). There was a substantial level of variability (I2 = 99%). The sensitivity analysis identified the research by Yang [16] as the cause of the heterogeneity. The 12-month postoperative FSH level remained considerably lower in the UAE group compared to the hysterectomy group after excluding Yang’s data (p < 0.00001). The Egger’s test showed a minimal probability of publication bias (p = 0.057).
LH
All studies included in the analysis provided data on preoperative LH levels. The combined preoperative LH levels were similar between the groups undergoing UAE and hysterectomy (MD = –0.12, 95% CI: –0.43–0.20, p = 0.47, Table IV). The heterogeneity was not statistically significant (I2 = 12%). The Egger’s test showed a minimal likelihood of publication bias (p = 0.527).
Table IV
Pooled results of pre- and postoperative LH
Four studies examined the LH levels 3 months after surgery [11, 12, 16, 17]. The combined LH levels at 3 months post-surgery were similar across the UAE and hysterectomy groups (MD = 1.01, 95% CI: –3.18–5.20, p = 0.64, Table IV). There was a substantial level of variability (I2 = 98%). The sensitivity analysis failed to identify the origin of heterogeneity. The Egger’s test showed a minimal probability of publication bias (p = 0.288).
Five studies analyzed the LH levels 6 months after surgery [12–16]. The combined LH level at 6 months post-surgery was notably lower in the UAE group compared to the hysterectomy group (MD = –3.09, 95% CI: –4.72– –1.46, p = 0.0002, Table IV). There was a substantial level of heterogeneity (I2 = 93%). The sensitivity analysis failed to identify the origin of heterogeneity. The Egger’s test showed a minimal probability of publication bias (p = 0.173).
Five studies examined the 12-month postoperative LH levels [12–16]. The combined 12-month postoperative LH level was significantly lower in the UAE group than the hysterectomy one (MD = –4.05, 95% CI: –5.2– –2.81, p < 0.00001, Table IV). There was a significant level of heterogeneity (I2 = 90%). The sensitivity analysis failed to identify the origin of heterogeneity. The Egger’s test showed a minimal likelihood of publication bias (p = 0.166).
E2
All studies included in the analysis provided data on preoperative E2 levels. The combined preoperative E2 levels were similar between the groups undergoing UAE and hysterectomy (MD = 0.43, 95% CI: –0.42–1.28, p = 0.32, Table V). The heterogeneity was not significant (I2 = 0%). The Egger’s test showed a minimal likelihood of publication bias (p = 0.557).
Table V
Pooled results of pre- and postoperative E2
Four studies examined the E2 levels 3 months after surgery [11, 12, 16, 17]. The combined E2 level at 3 months post-surgery was significantly higher in the UAE group compared to the hysterectomy one (MD = 143.87, 95% CI: 101.46–-186.29, p < 0.00001, Table V). There was a substantial level of heterogeneity (I2 = 100%). The sensitivity analysis failed to identify the origin of heterogeneity. The Egger’s test showed a minimal probability of publication bias (p = 0.3).
Five studies examined the E2 levels 6 months after surgery [12–16]. The combined E2 levels at 6 months post-surgery were similar across the UAE and hysterectomy groups (MD = 28.51, 95% CI: –2.80–59.82, p = 0.07, Table V). There was a substantial level of heterogeneity (I2 = 100%). The sensitivity analysis failed to identify the origin of heterogeneity. The Egger’s test revealed a substantial probability of publication bias (p = 0.003).
Five studies documented the 12-month postoperative E2 levels [12–16], and the combined 12-month postoperative E2 levels were similar across the UAE and hysterectomy groups (MD = 35.16, 95% CI: –12.25–82.58, p = 0.15, Table V). The heterogeneity was significant with an I2 value of 100%. The sensitivity analysis revealed that the research by Yang [16] was the cause of the heterogeneity. The 12-month postoperative E2 level was considerably higher in the UAE group compared to the hysterectomy group after excluding Yang’s data (p < 0.00001). The Egger’s test revealed a significant probability of publication bias (p = 0.041).
Discussion
Uterine myoma is a noncancerous tumor in the uterus that often occurs in females aged 30–50 years [15]. The cause of uterine myoma remains uncertain, with numerous experts suggesting a possible link to aberrant estrogen levels [18–21]. Surgical resection is the definitive therapy for uterine myoma. However, hysterectomy or myomectomy often leads to the removal of the ovarian branches of the uterine arteries. Some studies have suggested that the amount of blood flow to the ovaries would decrease by 50–70% after a hysterectomy [15]. Decreasing the blood flow to the ovaries will lead to a decrease in ovarian functioning. Fong et al. [22] discovered that patients lost over 87% of their primordial and developing follicles after a hysterectomy.
Recently, the approach to treating uterine myoma has changed. When treating the uterine myoma, it is important to protect the uterus. The UAE technique is a safe and efficient therapy for uterine myoma [23]. This meta-analysis revealed a substantial reduction in both post-UAE uterus and uterine myoma volume compared to pre-UAE measurements. Nevertheless, UAE might impact ovarian functions by potentially obstructing the ovarian microvasculature, leading to ischemia and a decrease in the primordial follicular pool [24].
Previous research has examined the impact of UAE on ovarian function. However, the findings are still debated. Czuczwar et al. [24] conducted a study comparing the effects on postoperative ovarian function of UAE, ulipristal acetate, and supracervical hysterectomy. The results indicated that the UAE had the most significant influence on ovarian function. The study participants were only monitored for 3 months. Hehenkamp et al. [25] found that both hysterectomy and UAE had a comparable impact on the ovarian function of elderly women transitioning into menopause. Younger women’s ovaries, however, could have a higher chance of healing after UAE [26].
The pooled preoperative FSH, LH, and E2 levels were comparable across the UAE and hysterectomy groups in this meta-analysis, confirming the validity of the research. Three months after surgery, the UAE group had comparable FSH and LH levels to the hysterectomy one, but with a considerably elevated E2 level. These results suggest that UAE and hysterectomy have a comparable effect on the short-term postoperative function of the ovary. A hysterectomy might result in worse long-term postoperative ovarian conditions than UAE, as shown by the 6- and 12-month postoperative outcomes, which revealed that the UAE group had considerably lower FSH and LH levels than the hysterectomy group.
Some researchers suggested that ovarian function may gradually return following UAE [15]. Recovery of ovarian function may occur due to the establishment of collateral circulation after UAE [15]. Several researchers have expressed the opinion that the impact of UAE on ovarian function in the UAE was reversible [23, 27].
There are limitations to this meta-analysis. Some of the studies included were retrospective, which might have introduced bias. Furthermore, several of the studies did not do follow-ups at 3, 6, and 12 months. Therefore, the combined findings of the postoperative outcomes need to be confirmed. Furthermore, as all the research analyzed was carried out only in China, future meta-analyses should aim to include data from other countries.