INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common form of entrapment neuropathy, affecting approximately 4% of the general population [1]. In CTS, the median nerve is compressed within the carpal tunnel underneath the transverse ligament. Compression results in paresthesias and pain, located in the hand and wrist, especially in the median nerve innervated area, usually with nocturnal exacerbation. In the advanced stages, atrophy and weakness of the thenar musculature become visible [2]. CTS is associated also with other less frequently investigated symptoms. One of such these is the disordered sleep associated with pain and paresthesias, which intensify at night due to increased pressure in the carpal tunnel as a result of the prolonged wrist flexion [3]. Another reason for nocturnal exacerbation may be the increased pressure of interstitial fluid due to lying in the horizontal position, which may decrease the lumen of the tunnel [4]. In consequence, patients with CTS show disrupted sleep cycles, increased levels of arousal, decreased sleep efficiency and reduced REM and slow wave sleep [5]. Insomnia may affect up to 80% of patients with CTS [6]. A consequence of insomnia may be daytime sleepiness [7]. Both conditions are associated with poor quality of life, with elevated risk of accident and of a number of cardiovascular, endocrine, and neuropsychiatric diseases [8]. Despite this, changes in sleep quality were reported in just 1% of all studies on the outcome of CTS therapy [9] and excessive daytime sleepiness in CTS has been the subject of just a small number of papers [7, 10].
Diagnosis of CTS should include nerve conduction studies (NCS), which reveal a slowing of sensory conduction velocity (SCV) and prolongation of the distal motor latency (DML) of the affected median nerve. In more advanced stages, the amplitudes of the sensory nerve action potential (SNAP) and compound muscle action potential (CMAP) decrease or even disappear. On the contrary, in the early stage a direct comparison of median to ulnar or radial latency of SNAP may reveal disturbed conduction, while SCV of the affected median nerve may still be within normal limits [2]. The measurement of electrophysiological changes is particularly helpful in the assessment of disease progression and the outcome of therapies. The most widely used grading system includes six severity grades, where six refers to no disease and one refers to extremely advanced CTS [11].
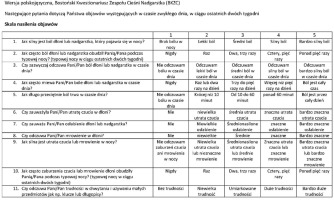
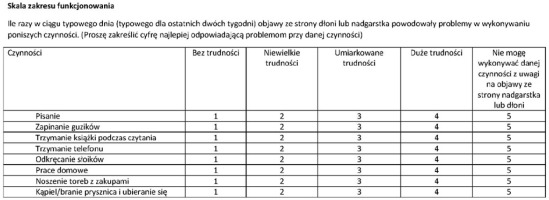
To systematize the clinical assessment of therapeutic outcomes a number of scales and questionnaires have been developed, of which the Boston Carpal Tunnel Questionnaire (BCTQ) is the most widely used [2, 12]. It consists of two self-reported subscales: the Symptom Severity Scale (SSS) and the Functional Status Scale (FSS). The first contains 11 items concerning the presence, frequency, and severity of typical CTS symptoms. The second consists of eight items in which patients grade the difficulties which may result from CTS during activities in daily life such as writing, buttoning, or opening jars. Each item is scored from one to five, where five refers to the worst severity of a given symptom in the SSS and an inability to perform a given task in the FSS. The total: score of the BCTQ and the scores from both subscales proved to be responsive to the outcomes of the therapeutic transection of the transverse ligament [13-18]. A systematic review showed its excellent psychometric properties, including focusing on the signs and symptoms most relevant for patients, as well as good construct validity, reflected by a close correlation with other, relevant outcome measurements [19]. Furthermore, the BCTQ showed strong internal consistency as well as good acceptability (only minimal burden for patients) and interpretability [19]. Following the establishment of its good psychometric properties the BCTQ has been translated into many languages [14, 20-25]. The published Polish language versions have to date either not been through the validation process [26] or the process was significantly incomplete, lacking a proper translation (which should include the participation of native speakers of the original language of the questionnaire and blinded, randomised backward translation), as well as testing for reactivity to treatment [27]. Therefore, for our own research as well as that of interested colleagues, one of the objectives of this study was to perform the adaptation and validation of the Polish language version of the BCTQ, including an investigation of reactivity of the questionnaire score to symptoms relief after treatment. The second objective was to investigate the severity of insomnia and daytime sleepiness in patients with CTS, and their changes after treatment.
MATERIAL AND METHODS
The study protocol was approved by the Ethics Committee of the Jagiellonian University (Permission No. 1072.6120.240.2018). All participants gave their written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Adaptation process
Translation and adaptation followed the recommendations of Guillemin et al. and of the American Academy of Orthopaedic Surgeons [28, 29]. The original was independently translated into Polish by two physicians (one neurologist and the other a pulmonologist, who was not familiar with the CTS in his practice), who were native Polish speakers with good acquired knowledge of English. The two translations were then randomly assigned to two medical students who were native English speakers with acquired knowledge of Polish (they grew-up in the United States and came to Cracow, Poland, to study) for backward translation. The backward translations were then compared with the original version, which until then had not been seen by either of the backward translators. A few deviations from the original in each backward translation were discussed among the study members to eliminate possible examples of non- equivalence. Following this discussion the Polish translation, which was used for pre-testing, was created. The pre-testing included ten patients with CTS. They filled out the questionnaire and discussed each item with a member of study team regarding its meaning, comprehension, and clarity. This process led to the creation of the final version, which was the subject of the validation process. This version is presented in Figures I and II.
Patients
For the consecutive sampling procedure 130 patients with CTS diagnosed in the EMG laboratory of the University Hospital in Cracow and at the EMG laboratory of Centrum Neurologii Klinicznej [Clinical Neurology Center] in Cracow were recruited. Inclusion criteria were age ≥ 18 years; presence of at least one of the typical symptoms of CTS, such as pain, paresthesias, numbness, or other unpleasant sensations in the hand or wrist, with exacerbation during the night; weakness of hand grip; or loss of hand dexterity. For inclusion, CTS also needed to be confirmed by the NCS. The exclusion criteria were treatment for CTS prior to inclusion, except for occasional pharmacotherapy. Further exclusion criteria were clinical or electrophysiological signs of peripheral neuropathy; diabetes; conditions in the patient’s history that may induce symptoms similar to CTS, such as cervical radiculopathy, myelopathy, or thoracic outlet syndrome; pregnancy and psychiatric conditions or cognitive impairment, which may interfere with participation in the study.
Measurements
The electrophysiological confirmation of a CTS diagnosis included findings such as a sensory conduction slowing of the median nerve, an increased median-ulnar latency difference in the sensory comparative test, a complete lack of sensory response, and a prolongation of distal motor latency of the median nerve with an active recording electrode placed over the abductor pollicis brevis (APB). In cases of a lack of response from the APB, an electrophysiological study of the fibres to the flexor pollicis longus was done, which documented normal conduction. Sensory fibres of the median nerve were studied antidromically using recording electrodes placed on the second (index) or the fourth (annularis) finger for comparative testing. The temperature of the skin of the investigated hand was maintained at approximately 32°C. NCS was performed with a Viking IV electromyograph (Nicolet Biomedical Incorporated, Madison, WI, USA) and 2-channel Medtronic Keypoint Portable equipment (Skovlunde, Denmark), using standard nerve stimulation, recording, and signal processing techniques [30]. The electrophysiologic severity of CTS was graded according to the approach of Padua et al. mentioned above [11]. Quality of life was assessed using the EQ-5D-5L quality of life questionnaire, which comprises a short descriptive system with 5 domains (EQ-5D-5L index) and a visual analogue scale (EQ-5D-5L VAS) [31]. The Athens Insomnia Scale (AIS) and Epworth Sleepiness Scale (ESS) were used to assess the presence and severity of insomnia and daytime sleepiness [32, 33]. AIS is an eight-item questionnaire which evaluates subjective sleep latency, awakenings during the night and in the morning, sleep time and quality, distress due to insomnia, and interference with daily functioning. Each item is rated from zero to three, where three refers to the most severe symptom of insomnia. The ESS contains eight items evaluating the chance of a respondent dozing in eight different everyday situations, such as reading, watching TV or sitting in a car in a traffic jam. Each item is rated from zero to three, where three refers to the highest chance of dozing. Patients filled out the pBCTQ and other scales and questionnaires at the same visit during which they were recruited. To evaluate the test-retest reliability, 26 of them filled out the pBCTQ once again, two weeks later. This subgroup was chosen randomly. The randomization list was created before prior to the study, using the website www.randomization.com. After the first visit, all included patients were contacted by telephone or asked during subsequent neurological ambulatory visits if they had undergone surgical decompression, rehabilitation, splinting, or injection for CTS. If so, they were asked to attend a control visit. For surgical decompression, the control visit was performed at least three months after the intervention. During the control visit the NCS was repeated, and the pBCTQ, other scales, and questionnaires were filled out.
Statistical analysis
The results were presented as mean and standard deviation. The pBCTQ was evaluated in terms of its internal consistency by calculating Cronbach’s α coefficient. Test-retest reliability was assessed by computing the intraclass correlation coefficients. Validity was examined using the intercorrelations (Pearson’s r) of SSS and FSS with EQ-5D-5L scores, the electrophysiological severity grade according to the CTS, and particular parameters of the NCS. Reactivity (responsiveness) was assessed by comparing the SSS and FSS scores before and after therapy using a t-test for related samples, and by measuring the effect size via Cohen’s d. Comparisons between pre- and post-treatment measurements were also performed for NCS data, CTS severity grade, ESS, and AIS. In the data collected before treatment, the ESS and AIS scores were correlated with NCS parameters and with CTS severity grade.
Calculations were made using the Statistica data analysis software system, version 13.0 (StatSoft, 2008; Palo Alto, CA, USA). The p < 0.05 was considered statistically significant.
RESULTS
Of the 130 patients recruited, 19 (14.6%) were male. Mean age was 53.1 ± 11.4. The mean SSS score was 3.05 ± 0.84, and FSS 2.63 ± 0.93. One (1%) patient was at grade 1 of electrophysiological severity of CTS; 18 (14%) were at grade 2; 82 (63%) at grade 3; 15 (12%) at grade 4; and 14 (11%) at grade 5. Thirty one of the patients had undergone surgery, three had received rehabilitation, and one had a nocturnal wrist splint. (According to patient reports, the COVID-19 pandemic contributed significantly to a relatively high proportion of untreated cases).
Cronbach’s α coefficients for the internal consistency of the pBCTQ was 0.91 for the SSS and 0.93 for the FSS. There was a strong correlation between SSS and FSS scores (Pearson’s r = 0.74, p < 0.001). The analysis of test-retest reliability showed no significant differences between the measurements separated by a two-week interval. At baseline, the mean SSS was 3.12 ± 0.73 and the mean FSS was 2.66 ± 0.81 points. The data obtained two weeks later were 3.00 ± 0.67 and 2.70 ± 0.83 (p = 0.419 and 0.803 respectively). The intraclass correlation coefficients were 0.69 for SSS and 0.55 for FSS. Respective standard errors of measurement were 0.47 and 0.62 and minimum detectable differences were 1.90 and 2.19. The overall validity of the pBCTQ was good, with significant correlations between both subscales and electrophysiological grading of CTS as well as EQ-5D-5L. (EQ-5D-5L was missing in two patients). The SSS and FSS also correlated with three of the four parameters of the NCS of the median nerve, namely, the SCV, DML, and CMAP amplitude. Furthermore, both subscales correlated with AIS and ESS scores. The results of the validity study and correlations with the AIS and ESS are presented in Table 1. Investigation of reactivity showed a significant reduction in both subscales after treatment. The SSS showed large and the FSS showed moderate clinical change (Table 2). Along with changes in the pBCTQ, a reduction in DML, EQ-5D-5L index, and AIS as well as an increase in CTS electrophysiological grading, EQ-5D-5L VAS, SCV, and amplitude of median SNAP were observed. (In two patients, DML was still prolonged after therapy, but SNCV normalized. The neurophysiological severity of CTS was set arbitrarily to 4 – the original classification does not include such a result). Electrophysiological assessments after treatment were missing for five patients.
Table 1
Correlations (Pearson) between subscales of pBCTQ and nerve conduction, quality of life, insomnia and daytime sleepiness
[i] DML – distal motor latency of the median nerve, CMAP – compound muscle action potential of the median nerve, SCV – sensory conduction velocity of the median nerve, SNAP – sensory nerve action potential of the median nerve, CTS – electrophysiologic grade of carpal tunnel syndrome severity, EQ-5D-5L index – summary index of EQ-5D-5L index score, EQ-5D-5l VAS – visual analog scale of EQ-5D-5L, AIS – Athens Insomnia Scale, ESS – Epworth Slepiness Scale
Table 2
Changes in pBCTQ and other outcomes after treatment (N = 31)
[i] M – mean, SD – standard deviation, d – Cohen’s d effect size, SSS – symptom severity scale, FSS – functional status scale, DML – distal motor latency of median nerve, CMAP – compound muscle action potential of median nerve, SCV – sensory conduction velocity of median nerve, SNAP – sensory nerve action potential of median nerve, CTS class – electrophysiological grade of the carpal tunnel syndrome severity, EQ-5D-5L index – summary index of EQ-5D-5L index score, EQ-5D-5l VAS – visual analog scale of EQ-5D-5L, AIS – Athens Insomnia Scale, ESS – Epworth Slepiness Scale
The calculation of correlations between pretreatment NCS parameters and CTS electrophysiological grading and AIS and ESS revealed no significant findings, except for a weak inverse correlation between AIS and amplitude of the SNAP of the median nerve. This analysis is shown in the Table 3.
Table 3
Correlations between electrophysiologic findings and sleep disorders and daytime sleepiness
| AIS | ESS | |||
|---|---|---|---|---|
| r | p | r | p | |
| DML | 0.139 | 0.113 | 0.089 | 0.310 |
| CMAP | –0.127 | 0.148 | –0.053 | 0.550 |
| SCV | –0.102 | 0.251 | –0.029 | 0.743 |
| SNAP | –0.178* | 0.042 | 0.017 | 0.848 |
| CTS class | –0.009 | 0.915 | –0.074 | 0.399 |
[i] AIS – Athens Insomnia Scale, ESS – Epworth Slepiness Scale, DML – distal motor latency of the median nerve, CMAP – compound muscle action potential of the median nerve, SCV – sensory conduction velocity of the median nerve, SNAP – sensory nerve action potential of median nerve, CTS – electrophysiological grade of carpal tunnel syndrome
DISCUSSION
The results indicate that our cross-cultural adaptation of the original English language questionnaire into Polish is reliable and valid. The internal consistency (Cronbach α was 0.91 for SSS and 0.93 for FSS) was equal to or exceeded that of the other language versions [14, 21, 24, 25]. The test-retest reliability was satisfactory, despite the fact that we had set long interval of two weeks separating the two administrations of the pBCTQ. A validation study of the Greek language version used, for example, a one-week interval [25] and of the Castellano language only two to three days [24].
The NCS and EQ-5D-5L were used to assess the validity of the pBCTQ. The first tool directly reflects the loss of function of the median nerve due to its chronic compression, which is the pathophysiological substance of CTS [2]. NCS served for validation also in the adaptation of the Portuguese, Spanish, Greek and Serbian language versions [21, 24, 25, 34]. The second tool assesses multidimensionally the quality of life and was also used during the adaptation of the Finnish language version [35]. The association of pBCTQ score with CTS severity and with resulting decrease of the quality of life was close: both subscales correlated with the CTS severity grade based on the NCS as well as with most of the NCS parameters. They also correlated with both parameters obtained from EQ-5D-5L: the summary index, a value from the calculation of the subscores from five tools assessing five different dimensions of the quality of life, and from the visual analogue scale, which expresses the general subjective health status in percentages.
Finally, the pBCTQ showed good responsiveness, as both subscales improved after interventions, the efficacy of which was confirmed by observed changes in NCS and quality of life.
Previous studies have reported decreased sleep quality in patients with CTS, as documented by questionnaires, actigraphy, and polygraphy. However, no direct correlation between the electrophysiological parameters of CTS and sleep disorders was found [6, 7]. Our data confirm these findings in a larger sample. In contrast, in our study insomnia and sleepiness showed significant correlation with both subscales of the pBCTQ. This discrepancy may be explained by the known resolution of nocturnal pain and numbness in the neurophysiologically advanced stages of CTS, when sensory fibres of the median nerve may lose their function, and the sleep-disturbing effect of CTS may decrease [36].
In our group, insomnia improved after treatment, which is in line with previous studies [7, 37, 38]. In the study of Lehtinen et al. which included 34 patients, daytime sleepiness was increased in patients with CTS in comparison to healthy controls, and it did not change after decompression, which however was performed in only six patients [7]. Our data confirms this last finding in a significantly larger sample and highlights the question of why sleepiness does not improve, despite a better quality of nocturnal sleep. We speculate that the residual symptoms of CTS may still cause awakenings, and that the objective quality of sleep, along with its restorative function, may improve less than the subjective satisfaction. This speculation is based on the results of Lehtinen et al., who observed a shortening of the objectively measured mean duration of nocturnal awakenings in patients operated on for CTS, but not a reduction of the number of awakenings. Moreover, in one (of six) of their patients an increase in sleep apnea episodes occurred after decompression. The alleviation of nocturnal pain in this patient probably allowed him to achieve deeper NREM and REM sleep, which are especially prone to sleep- disturbed breathing, which in turn is one of the main causes of excessive daytime sleepiness in the general population [8].
LIMITATIONS
We did not perform the comparison of the pBCTQ with Disabilities of the Arm, Shoulder, and Hand (DASH) or other similar tools, which evaluate the function and symptoms of the upper extremity. However, some other adaptations of the BCTQ, such as the Spanish or Turkish, similarly did not contain such a reference [24, 39]. On the other hand, our data contains the results of extensive electrophysiological examination, which objectively measures the severity of CTS and therefore is suitable for validation. Another limitation may be the relatively small proportion of patients assessed after treatment. This can be attributed, in part, to the COVID-19 pandemic, when many patients could not access surgery or other treatment. In the Chinese validation, however, a similar proportion of recruited patients were examined after treatment [23] and in the Cantonese, Spanish, and Greek adaptations none were [20, 24, 25].
CONCLUSIONS
The pBCTQ is a reliable, valid, and responsive questionnaire for the assessment of disease severity and therapeutic outcomes in Polish-speaking patients with CTS.
Insomnia and excessive daytime sleepiness are significant symptoms of CTS, and their severity correlates with both subscales of the pBCTQ.
In contrast to insomnia, daytime sleepiness may not improve after treatment.