Introduction
Herpesviridae is a family of viruses with over one hundred species, of which eight have been known to be pathogenic in humans. All these eight species present varying degrees of hepatotropism. The varicella-zoster virus (VZV), also known as human herpesvirus 3 (HHV3), is one of the viruses that belongs to this family. This exclusively human virus is responsible for causing two distinct clinical manifestations: chickenpox (varicella) and shingles (herpes zoster). Varicella is the manifestation of the primary infection with neuronal and haematogenous spread of the virus [1]. It usually presents as a pruritic, vesicular rash with fever. In immunocompetent children, the course of the disease is predominantly mild. However, in immunocompromised children the disease may be disseminated, with the involvement of crucial organs, presenting as hepatitis, encephalitis or pneumonia. A mild increase in transaminase levels has been recorded during varicella in otherwise healthy children [2], but fulminant hepatitis occurs primarily in immunosuppressed patients [3]. The aim of this study was to analyse the prevalence of liver involvement in primary VZV infection in immunocompetent children and to search for risk factors and clinical findings that may correlate with elevated liver enzymes.
Material and methods
We retrospectively analysed medical charts of children hospitalised between 2019 and 2022 (excluding the period of the COVID-19 pandemic, 2020-2021) due to varicella in our department. Inclusion criteria for this study were as follows: age 0-18 years, final diagnosis of varicella and assessed level of alanine aminotransferase (ALT). Exclusion criteria included known chronic liver disease. Age, sex, immunosuppression, source of the disease, comorbidities, clinical course, and laboratory findings were analysed. The diagnosis of varicella was based on its typical clinical presentation, regardless of whether there was a known exposure to a confirmed case of chickenpox. Other complications were diagnosed clinically or combined with laboratory findings or microbiological testing. Normal ranges of aminotransferase levels were assessed according to age using reference values of the Children’s Memorial Health Institute – the largest Polish paediatric healthcare institute with a child gastroenterology and liver transplant unit [4]. The diagnosis of hepatitis was based on the increase in serum ALT activity. Coagulation parameters were assessed according to age reference values [5]. We also assessed the body mass index (BMI) percentiles of studied patients and categorized them into groups of underweight, normal, overweight and obese according to the World Health Organization criteria. Patients’ BMI percentiles were analysed in order to exclude the potential influence of nonalcoholic fatty liver disease (NAFLD) on the observed levels of aminotransferases. Children were divided into two groups: with normal and elevated ALT levels.
Statistical analysis
Statistical analysis was performed using MedCalc Statistical Software version 22.007 (MedCalc, Ostend, Belgium, https://www.medcalc.org). Continuous variables were presented as the medians with interquartile ranges (IQRs) and were compared using the Mann-Whitney U test. Categorical variables were presented as numbers with percentages and were compared using the χ2 test. A two-sided p-value of < 0.05 was considered significant.
Ethical statement
Patient consent was waived due to the retrospective nature of the present study. The patients’ data were protected according to the European Union General Data Protection Regulation. The study was performed in accordance with the ethical standards presented in the 1964 Declaration of Helsinki and its later amendments.
Results
Study group
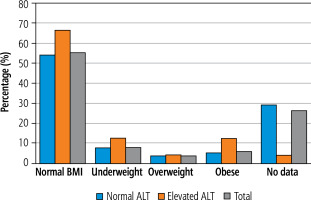
Medical records of 216 children hospitalised due to varicella between 2019 and 2022 (excluding years 2020 and 2021 due to the COVID-19 pandemic) were analysed. The baseline demographic characteristics of the study group are shown in Table 1. In 24 children (11.1%) ALT levels were elevated, whereas in 192 (88.9%) children, ALT levels were within the normal range. In 19 patients ALT levels exceeded the upper limit of normal (ULN) less than twofold, in 4 patients ALT levels were elevated 2-3-fold, and in only one infant the ALT value of 9.4× ULN was observed. Sex distribution was found to be similar in both groups and it was close to equal. The median age at the time of the diagnosis was significantly higher in the group of patients with elevated ALT: 5.5 years vs. 3 years in the group with normal ALT values (p = 0.02). The proportion of infants in both groups did not differ significantly and they constituted less than 20% of patients. In the whole cohort of this study, only 3 patients (1.4%) were immunosuppressed and none of them were in the group of patients with elevated ALT. We did not find a significant difference between the groups concerning BMI levels. In the group with normal ALT levels, patients with normal BMI constituted 54.2% (vs. 66.7% in the group with elevated ALT), underweight 7.8% (vs. 12.5%), overweight 3.6% (vs. 4.2%), and obese 5.2% (vs. 12.5%) (see Fig. 1, Table 1).
Table 1
Baseline demographic and epidemiologic characteristics of the study group
Fig. 1
Distribution of patients with normal and elevated alanine aminotransferase (ALT) according to the body mass index (BMI) in the course of varicella
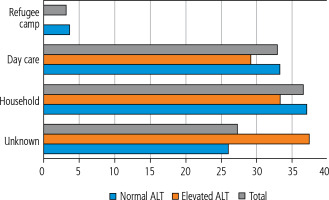
The source of infection was apparent in 74% of patients in the group with normal ALT levels and in 62.5% of patients in the group with elevated ALT levels. Household contacts constituted 37% in the first group and 33.3% in the second group, whereas day-care contact accounted for 33.3% and 29.2%, respectively. The full distribution of the source of infection is shown in Figure 2.
Fig. 2
Distribution of the source of varicella-zoster virus infection in the group with and without elevation of alanine aminotransferase (ALT)
Comorbidities were found in 21 patients (9.7%) – 15 patients belonged to the group with normal ALT levels and 6 to the group with elevated ALT levels. In the latter group, 4 out of these 6 cases were gastroenteritis. The number of cases of gastroenteritis was significantly higher in the group with elevated ALT levels (p = 0.006). Thus we can assume that in 4 out of our 24 patients coinfection with other viruses such as rotavirus might have influenced ALT levels.
Clinical findings
The clinical presentation is shown in Table 2. Children from the elevated ALT group were not found to be more severely ill. On admission, 87.5% of them were in good or fair general condition (compared to 86.9% in the group with normal ALT) and only 12.5% were categorized as in serious condition (vs. 13% in the other group). In the group without liver enzyme elevation, more patients were assessed as in good general status (p = 0.05). Most children were hospitalized due to bacterial superinfection of the skin lesions. Other reasons for admission to the hospital included: syncope, pneumonia, bronchitis, dehydration, reactive arthritis and coinfection with another pathogen such as influenza.
Table 2
Clinical presentation in the group with and without elevation of alanine aminotransferase during primary varicella-zoster infection
The median duration of fever and hospitalization was longer by 1 day in the group with elevated ALT. Accordingly, varicella lesions lasted longer in this group, 7.5 days versus 6 days in the group with normal ALT levels (p = 0.01). Although median C-reactive protein (CRP) (14.5 mg/l vs. 13.5 mg/l) and procalcitonin (PCT) (0.29 ng/ml vs. 0.37 ng/ml) values did not differ, median leukocyte values were lower (p = 0.01) in the group with elevated ALT (7.3 × 103/ml) compared to the other group (8.8 × 103/ml). Aspartate aminotransferase (AST) levels were increased in 8.9% of patients with normal ALT levels, whereas AST concentration exceeded the normal range in 83.3% of cases in the group with elevated ALT (p < 0.0001). Liver involvement did not result in abnormal coagulation testing, and neither prothrombin time nor international normalized ratio (INR) differed significantly between groups. None of our patients with varicella-induced hepatitis showed signs of liver failure. Liver ultrasonography was performed in 20% of patients in the group with normal ALT and in almost 30% of patients with elevated ALT. Enlargement of the liver was found only in 7.3% and 12.5% of patients, respectively (p = 0.37).
Discussion
Most articles on liver involvement during varicella focus on immunocompromised patients, in whom fulminant hepatitis has been reported [6]. Moreover, cases of varicella without or with very few skin lesions, but with visceral involvement, have been reported in this group of patients [7-9].
Little is known about the VZV hepatotropism in immunocompetent patients and its potential consequences. Elevation of aminotransferase levels has been reported in an immunocompetent adult patient who developed varicella after VZV vaccination [10]. In another case report, an immunocompetent adult patient had been admitted to the hospital with suspected acute pancreatitis and was later diagnosed with disseminated varicella infection [11]. Phuah et al. reported a case series of 8 patients, without known immunosuppression, who had a severe course of varicella. One of the patients developed disseminated VZV infection with hepatitis, disseminated intravascular coagulation and subsequent death [12]. Visceral VZV infection, although rare, might happen in immunocompetent children, and the scale of liver involvement during primary infection in children has not been widely studied.
According to Gershon, elevated aminotransferase levels in the course of varicella are common [2]. However, Feldman et al. stated that hepatitis defined as serum aspartate aminotransferase level ≥ 100 U/l) is infrequent in otherwise healthy children [13]. Both authors underlined that the condition is self-limiting and not followed by any sequelae. In our study group, we analysed levels of ALT with cut-offs established according to age-related reference values. These criteria were met by 11% of patients; thus, the frequency of liver involvement in varicella can be estimated as 1 in 10 paediatric patients. We also found a positive association of older age with higher prevalence of ALT elevation in older patients, which corresponds with the more serious course of varicella in older children. However, this association was not observed for infants, who are also considered to be in the risk group of more severe varicella. Sex distribution was equal. The source of infection defines the proximity and duration of contact with the infected person. The closer and the longer the contact is, e.g. home contact, the more viral particles can invade the organism. Higher viral load translates into more severe varicella and thus is an indication for acyclovir therapy [14]. However, we did not observe that the source of infection and thus viral load had an impact on liver involvement. According to Feldman et al., no correlation was found between the severity of chickenpox and liver involvement [13]. In our study, a higher percentage of children with normal ALT levels had good general condition upon admission compared to children with elevated ALT (borderline significant): 26% of children with normal ALT levels were found in good general condition vs. 8.3% in the group with elevated ALT levels, p = 0.05. However, there was no difference in the proportion of children admitted in serious condition (13% vs. 12.5%, p = 0.94). The median duration of fever, hospitalisation, and skin lesions were longer in the group with elevated ALT levels. Levels of inflammatory markers did not differ significantly, but the median leukocyte value was lower in children with liver involvement (8.9 vs. 7.3 × 103/mm3, p = 0.01). Due to its exclusiveness to the human species, comprehensive studies on the immunological background of VZV infection are limited. Studies on models using mice with severe combined immunodeficiency (SCID) and xenografts of human skin, thymus and dorsal root ganglia helped to shed some light on the pathology of infection. The major role of VZV-infected CD4+ T cells in spreading the infection throughout the organism has been proven, but the understanding of mechanisms, other than viral load, that would explain the number of lesions and visceral involvement from an immunological perspective remains vague [15]. It is not yet known whether the elevation of liver enzymes is due to viral replication in the liver or to the inflammatory response [1].
We did not observe differences in coagulation parameters between the groups. Only one out of 24 patients with hepatitis had an elevated INR (1.49), and it was probably attributable to a more severe clinical course with bacterial superinfection of skin lesions and suspected sepsis (PCT 3.28 ng/ml; lesions and hospitalisation lasted 9 days; concomitant treatment with 2 antibiotics).
The number of cases of gastroenteritis was significantly higher in the group with elevated ALT levels and was observed in 4 out of 24 patients (2 cases of rotavirus, 1 adenovirus, 1 unknown). Because of that, we cannot differentiate whether the observed rise in aminotransferase concentration in these 4 cases was attributable to the varicella-zoster virus or other viruses such as rotaviruses, which are also known to show some level of hepatotropism [16]. Due to this fact, the real incidence of liver involvement in primary VZV infection might be closer to 9%.
Overall, in our research, hepatitis occurred in 1 out of 10 patients. It was mild and did not result in liver failure. Patients with elevated ALT levels were older and had a longer clinical course of the disease. ALT levels were mostly less than twice the ULN. Overall, synthetic function of the liver was normal and ultrasonography revealed no abnormalities.
Our study is limited by the fact that it was conducted retrospectively. Therefore, not all children underwent ultrasonography examination or had their coagulation parameters taken. Moreover, we could not assess the time needed for normalization of liver parameters. Furthermore, the patients have not been tested for other hepatotropic viruses.
However, to the best of our knowledge, we present one of the few studies that describe the incidence of liver involvement in varicella in immunocompetent children. Moreover, we analysed multiple variables and assessed their correlation with the prevalence of VZV hepatitis.
Conclusions
During primary VZV infection around 1 out of 10 immunocompetent children experience mild hepatitis. Children in this group are usually older and tend to have a longer clinical course of varicella. Liver function testing and ultrasonography of the organ are generally normal. Liver failure was not observed.






