Introduction
Microscopic colitis (MC) is a disease that usually progresses with chronic watery diarrhoea in which the endoscopic imaging of the colon is normal [1]. There are histopathological abnormalities, and its diagnosis can only be conducted via colonic mucosal biopsy [2]. Microscopic colitis is divided into 2 sub-groups as collagenous colitis and lymphocytic colitis. Although these 2 sub-groups have similar gastrointestinal symptoms, there are histological differences between them. The diagnosis of lymphocytic colitis is performed by the detection of more than 20 intra-epithelial lymphocytes per 100 surface epithelial cells with an increased inflammatory infiltrate in the lamina propria of the colonic mucosa, while the diagnosis of collagenous colitis is made according to an increase in the thickness of the sub-epithelial collagen band > 10 µm [3, 4].
Celiac disease (CD) is an inherited disorder that occurs because of gluten intolerance found in grains such as wheat, barley, oats, and rye, and causes villi atrophy with inflammation in the small intestines. The clinical spectrum of CD is divergent. It can be completely asymptomatic or cause severe malabsorption syndrome. For this reason, the ages at which patients are first diagnosed are highly variable [5]. Auto-antibody titres are primarily checked in the diagnosis of CD, in suspected cases. Endoscopic biopsy from the second part of the duodenum is recommended in patients with positive auto-antibody levels or clinical suspicion thereof. The pathological evaluation is conducted according to Marsh classification [6]. In the treatment of celiac disease, a gluten-free diet should be given on a continuous basis. If the patient is not exposed to gluten, the inflammation cascade will not be active in the small intestine. A gluten-free diet reduces villi damage, thus improving clinical and laboratory findings [7]. Despite many studies, an effective medical treatment has not been developed yet [8].
In previous literature it was stated that the incidence of MC has been increasing gradually, especially in celiac patients. Individuals who do not respond to a gluten-free diet should be investigated in terms of MK. In addition to the fact that the age spectrums of CD and MC are quite different, CD is a genetically inherited disease.
Within the scope of this research, we have tried to elucidate biochemical, histological, and genetic traces of celiac disease in MC patients who responded and did not respond to treatment.
Material and methods
This was a prospective, cross-sectional, analytical study. The presence of celiac disease was investigated in 90 patients diagnosed with microscopic colitis during colonoscopy and biopsy due to chronic diarrhoea between September 2011 and December 2021.
Study protocol and ethical situation
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all participants. This study was approved by the Dicle University Faculty of Medicine Clinical Research Ethics Committee (ethics committee decision dated 20.09.2011 and numbered 177).
Inclusion criteria
Individuals ≥ 16 years old with a diagnosis of microscopic colitis in colonoscopic biopsy. Patients who agreed to give informed consent.
Exclusion criteria
Patients with celiac disease without microscopic colitis, subjects with malignities in and out of the colon. Patients with ulcerative colitis, Crohn’s Disease, or non-specific inflammatory bowel disease. Individuals with severe respiratory distress or heart failure, history of angina pectoris, and/or unstable angina. Subjects who were taking anti-coagulant treatment. Patients with active lower and upper gastrointestinal bleeding, who had undergone surgery/resection in thte stomach, duodenum, or jejunum. Patients who refused to give informed consent.
In addition to routine blood tests for the diagnosis of celiac disease, blood samples were taken for celiac autoantibodies and genetic testing. Upper gastrointestinal system endoscopy was recommended for histopathological examination of the bulbus and duodenum.
The diagnosis of celiac disease
Tissue transglutaminase antibodies
After fasting for at least 10 h, venous blood samples were taken from all individuals in the patient and control groups, and tTG IgA and tTG IgG antibodies (IMMCO 42 diagnostics ImmuLisaTM, Buffalo, NY, USA) were studied by micro-ELISA. Strips coated with human tissue transglutaminase were compared with plasma samples to be tested. The resulting colour change was transferred to numerical results by photometric analysis. Results of > 10 U/ml were considered positive in photometric analysis [9].
Anti-endomysium antibodies
Anti-endomysium IgA antibody (EMA-IgA, Dynex®, USA) was studied by micro-ELISA method. Strips coated with human tissue endomysium anti-bodies (as a commercial kit) were compared with a human plasma sample. In this test, if there were antibodies, the results were reported as titres, but results between 0 and 7 were considered negative and results above 7 were considered positive [10].
Anti-gliadin antibodies
Anti-gliadin antibodies were processed by micro-ELISA method (AGA-Ig A and Ig G Dynex®, USA). Plasma samples to be tested were compared with strips coated with human anti-gliadin antibodies. The resulting colour change was transferred into a numerical result by photometric analysis. In photometric analysis, 0–3 U/ml was considered normal, and results greater than 3 U/ml were considered positive [11].
Upper gastrointestinal endoscopy and duodenal biopsy
At least 4 biopsy samples were taken from the distal of the second part of the duodenum and 2 from the bulbus by performing an upper gastrointestinal system endoscopy with a video-endoscope. Samples were fixed in formalin solution for 24 h by preparing paraffin blocks after pathological routine procedures and 5-µm sections were prepared with a standard microtome. The sections were stained with Giemsa and haematoxylin/eosin as standard and examined under a light microscope at 200× magnification by the pathology specialists in our hospital. Duodenal mucosa biopsy specimens were evaluated according to the modified Marsh-Oberhuber classification [12].
Isolated intraepithelial lymphocytosis (in auto-antibody negativity) was not considered diagnostic for CD. Intra-epithelial lymphocytosis with crypt hyperplasia (Marsh II) or villous atrophy (Marsh III) was considered diagnostic for CD [13].
Genetic tests /HLADQ2 and HLADQ8
DNA was extracted from the blood collected in EDTA whole blood tubes with a DNA extraction kit according to the manufacturer’s instructions. Isolated DNA samples were measured by UV spectrophotometry at 260 nm absorbance and diluted with distilled water to the concentration required for polymerase chain reaction (PCR) (100 ng/µl). After amplification, celiac-specific HLA variants (HLA-DQA1*05, DQB1*02, and DQB*03:02 alleles) were examined. For visualization, 1.3% agarose stained with ethidium bromide was separated by electrophoresis, and the gel documentation system was used [14].
Evaluation of remission
Clinical remission after treatment in patients with MC was evaluated according to the Hjortswang criteria as recommended by the European Society of Gastroenterology (UEG). Patients with an average daily stool count of less than 3 and a watery stool for at most 1 day per week were considered to be in remission after at least 6 months of treatment [15].
The incidence of CD in MC patients was investigated by evaluating patients who were in remission and those who were not in remission according to auto-antibody levels, HLA positivity, and endoscopic biopsy results. In addition, the upper gastrointestinal systems of MC patients with and without remission were evaluated histopathologically.
Statistical analysis
The Kolmogorov-Smirnov test, Shapiro-Wilk test, coefficient of variation, skewness, and kurtosis methods were utilized to control the normal distribution of patient data. Mean and standard deviation values were expressed for continuous variables, while categorical variables were expressed as numbers (n) and percentage (%). Pearson’s χ2 test was used in the analysis of patients with microscopic colitis, who responded and did not respond to treatment. All tests were bilateral, and p < 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS24.0 for Windows (SPSS Inc., Chicago, IL, USA) software package.
Results
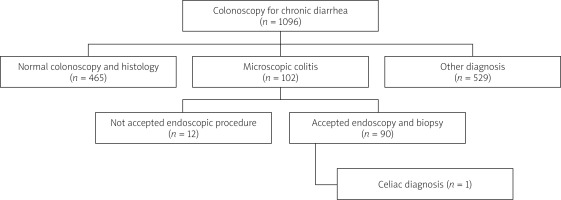
Out of 1096 patients who underwent colonoscopy due to chronic diarrhoea, 102 patients with histopathological diagnosis of microscopic colitis were included in the study. The rate of MC detection in patients who underwent colonoscopy for chronic diarrhoea was approximately 9.3%. No pathology was detected in the colonoscopies and biopsies of 465 (42.4%) patients with chronic diarrhoea. CD was detected in 1 (1.1%) patient among the MC patients who accepted endoscopic biopsy (Figure 1).
Of the MC patients, 86 (95.5%) had lymphocytic colitis and 4 (4.5%) had collagenous colitis. The mean age of the patients was 57.5 (28–79), and female gender (n = 73; 81.2%) predominated. While 48.8% (n = 44) of the patients did not use any medication, the most frequently used drugs among the patients were proton pump inhibitors (PPI), which were used by approximately one out of every 4 patients (n = 24; 26.6%). The most commonly used drugs after PPIs were anti-hypertensive drugs (18.8%) and NSAIDs (11.1%). The smoking rate among MC patients was 23.3% (n = 21). Two of the patients (2.2%) had CD in their first- or second-degree relatives.
While 77.7% (n = 70) of the patients diagnosed with MC responded to the treatment and their diarrhoea regressed, in 22.3% (n = 20) the desired response could not be obtained despite treatment with single and multiple drugs. The most frequently used drug (85%) in the treatment of MC was budesonide. In patients who did not respond to budesonide treatment, other drugs were used alone or in combination (Table I).
Table I
Demographic data, treatment responses, and drugs used in patients with microscopic colitis
Endoscopy was normal in 44.4% (n = 40) of MC patients, and endoscopic mucosal biopsy was normal in 25.5% (n = 23) of the patients. Endoscopically, gastritis (31.1%; n = 28) and oesophagitis (12.2%; n = 11) were the most common findings, while 55.5% of the patients showed different gastritis types histopathologically. The H. pylori positivity rate was 56.6% in MC patients. Although only 3 (3.3%) patients had inflammation in the bulb endoscopically, this rate was 17.7% (n = 16) histopathologically. One of the MC patients who underwent endoscopic biopsy was diagnosed with celiac disease (1.1%). No pathology was detected in the colonoscopy in 52.2% of the patients. The most common pathological findings in colonoscopy were polyps 24.4% (n = 22) and diverticulum 18.8% (n = 17) (Table II).
Table II
Endoscopic, histopathological, and colonoscopic findings of patients with microscopic colitis
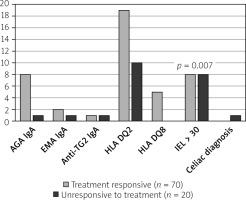
When MC patients were divided into groups of treatment responders and non-responders and evaluated in terms of celiac disease, the rate of AGA IgA positivity was 11.4% (n = 8) in MC patients who responded to treatment and 5% (n = 1) in non-responders. However, the difference between the 2 groups was not statistically significant (p = 0.359). Similarly, EMA IgA positivity was 2.8% (n = 2) in treatment-responding patients and 5% (n = 1) in treatment-resistant MC patients (p = 0.534). Likewise, no difference was found in terms of anti-TG2 IgA positivity in patients with and without treatment response (1.4% vs. 5%; p = 0.291). While HLA DQ2 positivity in MC patients was positive in 27.1% (n = 19) of responders, 50% (n = 10) of the non-responders were positive (p = 0.051). Although HLADQ8 positivity was 7.1% in treatment responders (n = 5), it was negative in non-responsive patients (p = 0.275). In mucosal biopsies taken from the duodenum of MC patients, the rate of IEL was higher in patients who did not respond to treatment (11.4% vs. 40%; p = 0.007). One of the patients who did not respond to treatment was diagnosed with celiac disease as a result of endoscopic biopsy (Table III).
Table III
Comparison of celiac disease-related autoantibodies, HLA levels, and inflammation in the duodenum in patients with microscopic colitis who responded and did not respond to treatment
We determined that the number of IELs was slightly more frequent in patients who did not respond to treatment when we examined the auto-antibody level bio-chemically and histopathologically at the mucosal level and with genetic parameters (HLADQ2 and HLADQ8). It was observed that the treatment response was not very determinative in terms of other parameters (Figure 2).
Discussion
In our study, we found the rate of MC to be 9.3% in patients with chronic diarrhoea. Although studies on this subject showed high heterogeneity, the probability of detecting MC in patients with chronic diarrhoea globally is 12.8% [16, 17]. We detected celiac disease in only 1 (1.1%) of the patients with MC and could not detect an increased frequency in MC patients. No increase was detected in celiac disease auto-antibody titres in responders and non-responders.
Although the general incidence rate of MC varies widely geographically, it is estimated to be around 119.4/100,000 [18, 19]. The incidence of MC seems to be directly related to age. A meta-analysis by Tong et al. found that pooled incidence rates were 4.14 and 4.85 per 100,000 person-years for collagenous colitis and lymphocytic colitis, respectively. In the same study, it was stated that MC was more common in women than in men. The median age of onset is 65 for collagenous colitis and 62 for lymphocytic colitis [20]. The increase in the incidence has been noteworthy especially in Europe and North America in recent years [21]. The most important reason for this increase could be the increased awareness and recognition of the disease in the last 40 years. There are no clear data on the prevalence of MC in Turkey.
In a meta-analysis the pooled global prevalence of CD based on blood test results was 1.4%, and the biopsy-confirmed prevalence was 0.7%. The prevalence of CD differs by continent, region, age, and gender. The highest prevalence rate has been reported in Europe [22]. Studies from Turkey indicate that the prevalence of CD is between 0.6% and 0.9%, and these rates are close to those of Europe [23]. In the study conducted in 2007, the frequency of celiac disease in our region was found to be 0.51% [24]. The majority of celiac patients are asymptomatic or have atypical symptoms, and this makes it difficult to determine the actual number of celiac patients.
When we examined the HLA tissue types in MC patients, we found that the distribution was not different from that of the normal population. HLADQ2 was positive in 31.8% of MC patients. While HLADQ2 positivity was 69.1% in celiac patients in a study from Turkey, it was found to be 28.4% in healthy volunteers [25]. In a study conducted in Denmark, 47.7% of the general population were HLADQ2 positive, while this rate was 89.9% in celiac patients [26]. This indicates the geographical heterogeneity of the disease. Additionally, it can be stated that MC was not associated with HLADQ2 alleles. Very strong HLADQ2 positivity is observed in CD but not in MC.
In a study conducted in Canada, the incidence of CD was found to be between 10.4 and 15.7 /100,000, the incidence of MC was found to be between 16.9 and 26.2/100,000 during a 5-year period, and it was stated that CD was more common in celiac patients. It has been stated also that there is a strong relationship between both diseases, and colonoscopic examination in terms of MC has been recommended, especially for celiac patients with continuous diarrhoea [27].
In a study of 46 patients with MC, no conclusion was drawn regarding the increase in celiac disease. On the other hand, in the same study, lymphocytic colitis was detected in 4 of 27 patients with CD [28]. In another study, CD was found in only one of 45 patients with CD who did not respond to treatment. Considering this low frequency of association, it was emphasized that there is no need for routine small bowel biopsy in MC patients [29].
The European Gastroenterology Association recommends screening for CD in MC patients [30]. However, CD has been diagnosed only bio-chemically in this population. In addition, most of these studies were retrospective, and only half of the target population could have been screened. These were probably MC patients who were unresponsive to treatment and whose diarrhoea continued [31–34]. The same guideline emphasizes that MC is not related to gluten.
Previous studies make it difficult for us to reach a clear judgment on this issue. For example, 32% of celiac patients have histological changes consistent with MC in the colon mucosa [35]. Another study states that MC was detected in 4.3% of celiac patients. This was about 70 times higher than the normal population. The same study stated that almost all MC patients had negative celiac autoantibodies [36]. In another study, 59 patients with MC were analysed, and it was stated that CD was detected in only 1 patient. The HLADQ2 level was higher in patients with lymphocytic colitis in this group [37].
One of the interesting results was the presence of increased intra-epithelial lymphocytosis in the small intestine samples of patients who did not respond to treatment. Although 17.7% of our patients were histologically positive for bulbitis and 56.6% for H. pylori, approximately 4 times more intraepithelial lymphocytosis was observed in patients who did not respond to treatment. However, these patients did not meet diagnostic criteria for CD both bio-chemically and according to the Marsh classification. Although there are studies that show increased IEL especially in the ileum in MC patients, more research is needed on this subject [38].
An important detail should not be overlooked here: There are studies showing that uncontrolled intra-epithelial lymphocytosis also affects the colon as a result of overexpression of IL-15 and MHC class-1 molecules in treatment-refractory celiac patients [39–41]. This may lead to confusion and an unnecessary diagnosis of lymphocytic colitis. The best way to distinguish this can be elaborated as the regression in diarrhoea and improvement of clinical findings with a gluten-free diet. In such cases the diagnosis can be changed to colonic lymphocytosis, especially in patients with celiac disease who do not comply with the treatment. Therefore, hasty colonic biopsies for MC may cause misleading results, especially in celiac patients who do not comply with the diet.
There are many question marks that need to be clarified in order to fully express the relationship between MC and CD. Celiac disease is a proven genetic disorder, but on the other hand, a genetic relationship has not been revealed for MC yet, and its pathophysiology has not been fully elucidated [42]. Many aetiological causes of this disease, including drugs and smoking, have been described [43, 44]. While most celiac patients are diagnosed in the first decades of life, the majority of MC patients present with symptoms and are diagnosed in their 50s and 60s. While there is no obvious gender difference in celiac patients, there is a clear predominance of female gender in MC patients.
The most important limitation of our study is the lack of a control group and its unidirectional nature. We only investigated the incidence of CD in MC patients. If we could also investigate the incidence of MC in a sufficient number of celiac patients, we could obtain much more data on the relationship between these 2 diseases. Our CD autoantibody positivity rates in MC patients were misleadingly high because the number of patients was not sufficient. For this, autoantibodies for celiac disease could be examined and compared in healthy volunteers. However, in order not to deviate from the purpose of our study, we focused only on patients with MC, but we plan to perform separate research on this.
Conclusions
In previous literature it was reported that the risk of MC was increased in celiac patients. From the results of our study, we can state that there was no increased risk for CD in MC patients. Based on this, we would like to point out that we could not reach results that could provide a strong recommendation for routine laboratory and endoscopic examination in terms of investigating CD in CD patients, leaving aside the MC patients who did not respond to treatment. Future genetic studies are required to clarify the relationship between these 2 diseases.