Uveitis is the fifth leading cause of blindness in developed countries [1, 2]. Epidemiological data show that uveitis currently affects over 2 million people worldwide [3]. According to data published by the Polish Association of the Blind in 2017, there are approximately 175,000 people with visual impairment secondary to uveitis living in Poland [4]. Uveitis affects all age groups, but most commonly, in 70-90% of cases, the condition develops in people aged between 20 and 60 years [2, 5].
In the majority of patients, the inflammation in uveitis is non-infectious (67 to 90% of all cases) [5]. It may accompany systemic autoimmune disorders or be confined to the organ of vision only, as in Fuchs uveitis syndrome [2, 3].
Ongoing inflammation and the development of local complications during the progression of the pathology can result in significant visual impairment. The complications include band keratopathy, cataract, glaucoma, post-inflammatory changes in the vitreous, vitreoretinal proliferations, inflammatory macular edema, and less commonly also exudative retinal detachment, and edema and atrophy of the optic disc [5]. One of the most serious complications of uveitis is glaucoma. It affects over 65% of patients with anterior uveitis, 13% with choroiditis, and 4% with intermediate uveitis [5, 6].
Despite the increasing use of immunosuppressive and immunomodulatory agents and biological therapy in the treat- ment of severe non-infectious uveitis, glucocorticosteroids remain the cornerstone of treatment [5]. In patients with uveitis, glucocorticosteroids may be administered systemically or topically; in the form of drops, periocular injections, intravitreal injections or implants, and via suprachoroidal delivery [7-11]. If topical glucocorticosteroids are found to be ineffective, there are indications for introducing oral or intravenous glucocorticosteroids in the form of pulse therapy with long-acting glucocorticosteroid agents [8, 9, 12]. However, irrespective of the route of administration, chronic glucocorticosteroid use is linked to the risk of serious ocular complications, including IOP elevation, glucocorticosteroid-induced glaucoma, cataract, delayed wound healing, poorer healing of corneal epithelial defects, and an increased risk of secondary bacterial, viral, and fungal infections [13-16].
It is important to highlight that the rise in the IOP and the development of secondary glaucoma in individuals with uveitis may be associated both with the inflammatory condition itself and prolonged glucocorticosteroid treatment, with the latter significantly elevating the risk [6, 17].
This article aims to provide a classification and review of the current state of knowledge of the pathophysiology and pharmacological management of patients with uveitis associated with elevated IOP.
SECONDARY UVEITIC GLAUCOMA
In 1813, Joseph Beer first reported the association between uveitis and secondary glaucoma in a patient with arthritic iritis [after 18]. It is currently understood that the complication occurs in 20-46% of patients with uveitis, most commonly as a complication of chronic anterior uveitis [6, 18-21]. A significant risk factor for the development of secondary uveitic glaucoma is age, with a higher occurrence rate in adults compared to children. In children, the most prevalent condition associated with secondary uveitic glaucoma is juvenile idiopathic arthritis (JIA) [22]. Uveitis of viral (herpetic) etiology is also recognized as a predisposing factor to an increase in the IOP and the development of uveitic glaucoma [18, 22].
Secondary uveitic glaucoma can take the form of secondary angle-closure uveitic glaucoma with or without pupillary block, secondary open-angle uveitic glaucoma, or uveitic glaucoma associated with trabeculitis [18, 20, 21].
Secondary angle-closure uveitic glaucoma
Secondary angle-closure uveitic glaucoma with pupillary block
The pathogenesis of this form of uveitic glaucoma is closely linked to the formation of posterior adhesions/synechiae. When the adhesions cover 360° of the circumference of the pupillary aperture, they disrupt the circulation of the aqueous humor. The obstruction of free flow of the aqueous humor from the posterior to the anterior chamber of the eye through the pupillary aperture results in elevated pressure in the posterior chamber. This causes the iris to bulge forward, leading to the formation of iris bombé and ultimately causing closure of the drainage angle. In such cases, treatment involves pharmacological removal of the posterior adhesions/synechiae using mydriatics including 1% tropicamide, 10% phenylephrine or 1% atropine. If the drops are ineffective, an adrenaline solution (0.1%, i.e. diluted at 1 : 1000) can be administered subconjunctivally [19, 20].
In addition to addressing the underlying cause of drainage angle closure, therapy also includes medications lowering the IOP. To prevent the formation of anterior (iridocorneal) adhesions, intensive topical glucocorticosteroid treatment is applied.
Secondary angle-closure uveitic glaucoma without pupillary block
This type of uveitic glaucoma develops in association with granulomatous inflammation. Granuloma forming at the base of the iris, close to the anterior chamber angle, and subsequently shrinking can lead to the closure of the drainage angle.
The treatment of patients presenting with this form of glaucoma is similar to that prescribed in cases of secondary closed-angle uveitic glaucoma with pupillary block [19, 20].
TRABECULITIS
Trabeculitis, which is most commonly caused by viral infections (Herpes simplex and Herpes zoster), is routinely treated with anti-inflammatory drugs (glucocorticosteroids) in combination with antiviral medications and IOP-lowering agents [19-21]. Antiviral agents can be used topically and systemically.
STEROID-INDUCED OCULAR HYPERTENSION AND STEROID-INDUCED GLAUCOMA SECONDARY TO UVEITIS
Steroid-induced glaucoma (SIG) was first reported by Gordon and McLean in 1951 in a patient treated with adrenocorticotropic hormone (ACTH) [23]. It is currently recognized that IOP elevations occur most commonly in patients treated with topical glucocorticosteroids in the form of eye drops, periocular injections, and intravitreal medications. In a smaller proportion of cases, SIG arises from the use of glucocorticosteroids administered intranasally, via inhalation, in systemic formulations, as well as in dermatological ointments and creams [24-27].
Elevated intraocular pressure in response to glucocorticosteroid therapy may develop after several weeks, typically between 2 to 6 weeks after treatment initiation, though occasionally it may occur even earlier, within a few days of starting the treatment. An early increase in the IOP is observed in individuals who are highly sensitive to glucocorticosteroids [24, 25]. Nonetheless, cases of late IOP elevation, occurring even a few months after intravitreal glucocorticosteroid administration, have also been reported in the literature [24, 28].
If a glucocorticosteroid-induced rise in the IOP is not promptly diagnosed, persistently elevated IOP can lead to the development of secondary steroid-induced glaucoma. If left untreated, the condition may result in irreversible visual impairment [25].
Individual response to glucocorticosteroids is determined genetically. Patients responding to glucocorticosteroid treatment with an increase in the IOP are referred to as steroid-responders.
In 1965, Armaly and Becker proposed three categories of steroid responsiveness, based on the degree of IOP increase after use of dexamethasone and betamethasone eye drops [29]:
high steroid responders (5% of the population) who develop an IOP greater than 31 mmHg or an IOP rise of more than 15 mmHg from baseline;
moderate steroid responders (30% of the population) who develop an IOP between 25 to 31 mmHg or an IOP rise of 6 to 15 mmHg from baseline;
steroid non-responders (65% of the population) who have an IOP lower than 20 mmHg or an IOP rise of less than 6 mmHg from baseline. In this group of individuals, this slight increase in the IOP may occur after an extended period of glucocorticosteroid use.
Pathophysiology
Steroid-induced ocular hypertension (SIOH) is attributed to overexpression of α receptors in the nuclei of cells making up the trabecular meshwork [30, 31].
The nuclei of these cells contain two types of glucocorticosteroid receptors: α and β. β receptors suppress the activity of α receptors, which play a role in the synthesis of glycosaminoglycans and the extracellular matrix. In the eyes with steroid-induced glaucoma, β receptors do not function properly, resulting in α receptors escaping their regulatory control [30, 32]. The increase in the number and activation of α receptors under the influence of glucocorticosteroids upregulates the expression of proteins, including fibronectin, glycosaminoglycans, elastin, and type IV collagen, in the extracellular matrix. In addition, glucocorticosteroids suppress phagocytosis, which increases the accumulation of the proteins enumerated above in the peritubular space of the trabecular meshwork [32, 33]. All these processes contribute to a reduction in the number of cells forming the trabeculum, while simultaneously enlarging the volume of extracellular deposits. This, in turn, is the primary factor responsible for a greater obstruction to the outflow of the aqueous humor and an increase in the IOP [30, 31, 33].
Steroid-induced glaucoma is a genetically conditioned complication. Researchers have identified a total 48 different polymorphisms across 33 genes, which are likely responsible for IOP elevation associated with glucocorticosteroid treatment. Among these, there are polymorphisms of genes encoding myocilin, α 1-antichymotrypsin, pigment epithelium-derived factor (PEDF), corneal-derived transcription factor 6, and prostaglandin D2 synthase [33, 34]. The most important and, at the same time, one of the first and best-studied genes linked to the development of glaucoma is the MYOC gene, formerly known as TIGR (trabecular meshwork-inducible glucocorticoid response protein). Mutations in this gene are detected in 2-4% of individuals with primary open-angle glaucoma (POAG), 10-22% of those with familial POAG, and 8-20% of individuals with juvenile POAG [34, 35]. The MYOC gene is responsible for encoding a protein known as myocilin which, in response to glucocorticosteroids, is strongly expressed on trabecular cells, in the iris, and in the ciliary body. Myocilin expression has been found to be linked to steroid-induced IOP elevation. However, there are also reports that raise doubts about the association between the TIGR gene and IOP elevation after steroid therapy [36].
Risk factors for steroid-induced glaucoma
In addition to genetic factors contributing to the development of steroid-induced glaucoma, there are various local and systemic factors that are known to be predictive of the development of this condition.
One of the major contributors to the development of SIG is POAG, with as many as 80-90% of cases exhibiting IOP elevation induced by glucocorticosteroids [6, 25]. Other risks factors include a positive family history of POAG, high myopia, and secondary glaucoma associated with uveitis itself [6, 30]. An increased risk of elevated intraocular pressure following glucocorticosteroids treatment has also been noted in patients who have undergone penetrating keratoplasty, especially in cases of Fuchs’ dystrophy and keratoconus [6, 32]. Systemic conditions associated with an elevated risk of SIG include diabetes mellitus and connective tissue disorders [32]. Steroid-induced glaucoma is also more prevalent in two age groups: elderly patients and children under the age of 6 [6, 17].
Another crucial risk factor for IOP elevation accompanying glucocorticosteroid therapy is treatment with high-potency glucocorticosteroids, such as prednisolone and dexamethasone [6, 37].
CHOOSING THE OPTIMAL GLUCOCORTICOSTEROIDS FOR MANAGING PATIENTS WITH UVEITIS AND ELEVATED IOP
The efficacy of anti-inflammatory treatment with glucocorticosteroids administered topically depends on multiple factors, including the potency of the medication, its ability to penetrate the cornea, concentration, formulation type, and the frequency and way of application by the patient. Relative anti-inflammatory potency of glucocorticosteroid eye drops versus hydrocortisone, both in vitro and in vivo, is shown in Table I, based on data published by Samudre et al. [38].
Table I
Relative anti-inflammatory potency of steroid eye drops versus hydro- cortisone in vitro and in vivo, based on data published by Samudre et al.
The efficacy of a given glucocorticosteroid in the treatment of uveitis depends not only on its anti-inflammatory potency but also on the capacity to penetrate the cornea. Awan et al. demonstrated that among glucocorticosteroid eye drops, the highest concentration in the human aqueous humor is achieved with 1% prednisolone solution (in Poland, 0.5% prednisolone acetate suspension is available) and 0.1% dexamethasone solution, which is why they are the preferred first-line glucocorticosteroids for uveitis treatment [39]. Figure 1 shows the peak concentrations in the aqueous humor of different glucocorticosteroids administered topically. Prednisolone acetate solution achieved the highest concentration in the aqueous humor followed by dexamethasone-based medications (note that dexamethasone-cyclodextrin complex is not available in Poland), whereas fluorometholone drops exhibited the lowest penetrability into the anterior chamber [39].
Figure 1
Peak concentrations in the aqueous humor of steroids administered topically according to Awan et al. (Courtesy of Prof. Amer Awana, Shifa College of Medicine, Shifa Tameer-e-Millat University, Islamabad – Pakistan)
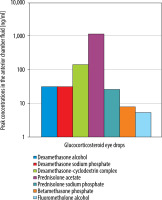
Taking into account the various dosage forms of the same glucocorticosteroid (i.e. suspension or solution), clinical observations demonstrated the superiority of drugs administered in the form of solutions over suspensions. The advantage is attributed to the consistency of dose in each drop of the drug administered to the patient. With suspensions, there is no assurance that the prescribed dose of the medication will be delivered with every single application. To mitigate the effects of this phenomenon, it is advisable to shake the bottle before each application of eye drops in the conjunctival sac. Furthermore, there is an increased risk of reduced tolerance to suspension-based drugs due to the presence of irritating solid-phase particles [40].
Taking into account all the aspects outlined above, is there an optimal glucocorticosteroid formulation for treating uveitis? The optimal glucocorticosteroid for patients with uveitis must possess high anti-inflammatory potency and achieve an appropriate concentration in the aqueous humor without triggering an IOP increase. In addition, the formulation must ensure that a consistent and unchanging dose is delivered with each drop [37, 38].
The anti-inflammatory potency of glucocorticosteroids, as well as the dosage frequency and duration of glucocorticosteroid therapy, correlate with the potential of glucocorticosteroids to increase the intraocular pressure. The risk of SIOH during treatment with glucocorticosteroids characterized by high potency and good penetration into the aqueous humor, i.e. dexamethasone and prednisolone, is high, whereas the likelihood of IOP elevation during therapy with glucocorticosteroids showing lower anti-inflammatory potency compared to the above-mentioned drugs (i.e. fluorometholone or loteprednol) is low [40, 41]. It needs to be highlighted, though, that the low risk of inducing an IOP increase correlates with the limited ability of the latter two glucocorticosteroids to penetrate the cornea. Consequently, they are less effective in controlling anterior uveitis [41].
The time after which an increase in the IOP is observed varies, depending on the properties of the glucocorticosteroid used. Glucocorticosteroids with high anti-inflammatory potency may induce an increase in the IOP within the first few weeks of treatment, while weaker glucocorticosteroids might cause IOP elevation only after several months from initiating therapy [42].
In patients with chronic anterior uveitis who need long-term treatment with glucocorticosteroids-based eye drops (often for several months), it may be advisable to use a preservative-free glucocorticosteroid, such as Dexafree (dexamethasone phosphate). Prolonged use of eye drops containing preservatives can lead to eye irritation, allergy symptoms, and dry eye.
MANAGEMENT OF PATIENTS WITH UVEITIS RECEIVING TOPICAL GLUCOCORTICOSTEROID THERAPY
A baseline measurement of the intraocular pressure should always be obtained prior to initiating glucocorticosteroid treatment. After starting topical therapy with glucocorticosteroid eye drops, reassessment of the intraocular pressure around two weeks later is recommended. Subsequently, regular monitoring every 4 weeks for 2–3 months is advised, followed by reassessments every 6 months if the therapy is to be continued. This monitoring schedule applies to patients whose IOP values during the follow-up are normal. If a trend towards an elevation in the IOP is observed, the frequency of follow-up visits should be adjusted on an individual basis.
Following intravitreal glucocorticosteroid injection or insertion of implant with a long-acting glucocorticosteroid, it is advisable to measure the IOP 30 minutes after the procedure, followed by measurements after 1 and 2 weeks, and then monthly for a duration of up to 6 months [28].
An increase in the IOP induced by glucocorticosteroids administered in the form of eye drops typically occurs after 2-4 weeks of treatment. Less commonly, there may be an acute response to the glucocorticosteroid, manifesting as an increase in the IOP within the first few hours of commencing dexamethasone treatment [43]. After intravitreal glucocorticosteroid injection, an increase in the IOP is observed in glucocorticosteroid-sensitive patients, similarly to the response observed after eye drops, 3-4 weeks after injection, but cases of IOP elevation several months after the procedure have also been reported. In patients with pseudophakia and post vitrectomy, who are administered a glucocorticosteroid intravitreally, an increase in the IOP may occur much earlier [44]. However, it is important to note that both the IOP elevation and the time when it occurs are influenced by the type of glucocorticosteroid used for treatment and its dosage. Following intravitreal injection of triamcinolone, an increase in the IOP develops in up to 50% of patients. After dexamethasone or fluocinolone implant injection, IOP elevation was observed in 26.9-41.5% and 11-38.4% of patients, respectively [45, 46].
A rise in the intraocular pressure observed after treatment with glucocorticosteroid eye drops does not necessarily indicate that the same response will occur after using other glucocorticosteroids, in different dosage forms, administered via other routes. Nonetheless, studies found that an increase in the IOP was more common in patients treated with glucocorticosteroids administered via periocular injection compared to glucocorticosteroid medications applied to the conjunctival sac [47]. In addition, IOP elevation is more frequently observed after the administration of glucocorticosteroid eye drops than during systemic glucocorticosteroid treatment [15, 45]. In the latter case, it is advised that patients on chronic oral treatment with prednisolone at a dose of ≥ 10 mg/day should have their IOP checked at 1, 3, and 6 months of the therapy, followed by assessment every 6 months thereafter [45].
If the IOP increases during glucocorticosteroid therapy, various management options are available. One of them is to discontinue the glucocorticosteroid. Following discontinuation of glucocorticosteroid therapy chronic glucocorticosteroid response usually resolves in 1-4 weeks, while acute response may subside within a few days of cessation of glucocorticosteroid therapy. In cases where glucocorticosteroid therapy has been used for a duration of 18 months or more, IOP elevation may persist even after discontinuing the medication. Literature reports suggest that in approximately 3% of cases, the response to glucocorticosteroids may be irreversible [42]. In practice, however, it is often impossible to discontinue the glucocorticosteroid completely. Typically, the treatment involves reducing the dose or dosage regimen of the glucocorticosteroid, or substituting the glucocorticosteroid with an alternative glucocorticosteroid agent associated with a lower risk of IOP elevation (fluorometolone, loteprednol) [37, 40]. Other options to address the issue involve substituting the glucocorticosteroid with a non-steroidal anti-inflammatory drug (NSAID) or reducing the glucocorticosteroid dose and adding an NSAID to the treatment [48]. Nonetheless, the control of uveitis is rarely possible with NSAIDs in monotherapy. If the IOP increases during therapy with systemic glucocorticosteroids, the recommended course of action is to reduce the dose of the glucocorticosteroid and subsequently replace it with an immunosuppressive or immunomodulatory drug. If these measures prove ineffective, biological therapy may be considered [8, 48].
Even though secondary SIG is a dangerous complication of glucocorticosteroid therapy, and poses a significant risk to vision, the prognosis can still be favorable, provided that the condition is diagnosed early and anti-glaucoma treatment is initiated promptly [25].
The first-line drugs in the therapy of SIG in patients with uveitis are β-blockers and carbonic anhydrase inhibitors [30, 31]. The latter are used in the form of eye drops and/or oral medications. α 2-agonists are classified as second-line drugs; they can be used in monotherapy or in combination with other medications from the first-line treatment group [30, 31].
Prostaglandins are relatively contraindicated in patients with uveitis because of their association with an increased risk of macular edema and the potential to promote intraocular inflammation [30, 49].
Hyperosmotic drugs have limited utility as well, with their therapeutic efficacy restricted by impairment of the blood-retinal barrier in uveitic eyes [30, 31].
Miotics are strictly contraindicated, as they can exacerbate damage to the blood-anterior chamber barrier and promote the formation of posterior adhesions/synechiae [30].
High hopes are being pinned on emerging therapies. One of the promising treatments for SIG is netarsudil, an Rho kinase inhibitor. Experimental studies demonstrated its significant potential in reducing the intraocular pressure by restoring the outflow of aqueous humor through the trabeculum in mice [50].
When pharmacological methods to reduce the IOP in uveitic eyes are unsuccessful, anti-glaucoma laser treatments may be considered, and if these fail to produce satisfactory results, surgical interventions become an option [51]. In patients with angle-closure uveitic glaucoma with pupillary block, peripheral laser iridotomy can be an effective and safer choice when compared to surgical iridectomy particularly in cases of active uveitis [51]. Selective laser trabeculoplasty (SLT) is used in the treatment of secondary glaucoma, both uveitic glaucoma and SIG. However, the procedure is absolutely discouraged in cases of uveitic glaucoma in eyes with active inflammation [52, 53]. Cyclodestructive treatments (cyclophotocoagulation, cyclotherapy) may exacerbate inflammation, lead to hypotony and ocular atrophy. For this reason, cyclodestructive procedures should only be considered as a last choice procedure after other treatment modalities have proven ineffective or unsatisfactory [51]. Anti-glaucoma surgical interventions for treating uveitic glaucoma include glaucoma drainage devices with drainage implants, such as the Ahmed valve or Baerveldt implant [51, 54, 55]. Standard trabeculectomy or a combined procedure with stent implantation supplemented with antimetabolites (5-fluorouracil or mitomycin C) showed efficacy in reducing scarring and lowering the risk of surgical failure in eyes with uveitis [54, 55]. Minimally invasive anti-glaucoma procedures are also increasingly employed to treat secondary uveitic glaucoma. Ab interno trabeculotomy increases the outflow of the aqueous humor by removing part of the trabecular meshwork, which may be blocked, for example, by inflammatory cells [51, 54]. Canaloplasty is also generating significant interest because it expands Schlemm’s canal and stretches the peritubular space of the trabecular meshwork in which, as a result of chronic glucocorticosteroid therapy, overexpression of proteins in the extracellular matrix occurs, leading to an increased resistance to the outflow of the aqueous humor [53, 55].
CONCLUSIONS
Both active uveitis and IOP elevation associated with uveitis itself and/or glucocorticosteroid therapy may lead to irreversible vision damage.
In view of the increased risk of IOP elevation in patients with uveitis undergoing glucocorticosteroid treatment, it is important to conduct regular IOP monitoring in this patient population.
Following IOP elevation in patients with uveitis, treatment adjustments are necessary in order to maintain a balance between normalizing the IOP and maintaining full control of inflammation. In such situations, it is advisable to reduce the dosage of the glucocorticosteroid or switch to a glucocorticosteroid agent associated with a lower risk of inducing an increase in the intraocular pressure. Additionally, conservative anti-glaucoma treatment should be initiated.
When pharmaceutical methods are insufficient to control the intraocular pressure, laser procedures (peripheral iridotomy, trabeculoplasty), as well as anti-glaucoma surgical interventions, should be considered in the affected eyes [30, 31].

 ENGLISH
ENGLISH




