INTRODUCTION
Cataract surgery is currently the most frequently performed surgical procedure globally [1]. As human lifespans increase, so do their expectations for the quality of vision [2]. The pursuit of modern solutions in intraocular lens technology is consistently ongoing. A wide range of implants are now available to correct refractive errors and presbyopia [3]. Most of them are designed for intracapsular implantation, which is considered the standard of care [4]. However, an increasing number of IOLs designed for implantation in the ciliary sulcus are also being introduced to the market. They are most commonly used for the correction of postoperative residual refractive errors [4, 5], but also find application in the treatment of postoperative presbyopia [7, 8], adverse photic phenomena [9, 10], high astigmatism secondary to penetrating corneal transplant surgery [11, 12], as an optical aid in the treatment of age-related macular degeneration [13], and recently even in the management of diabetic retinopathy [14]. Furthermore, they have been successfully employed in children as part of the surgical treatment of congenital and juvenile cataracts [15, 16], as well as in refractive surgery, where a suitable type of implant is placed in the sulcus in the phakic eye [17].
Presbyopia-correcting IOLs, including multifocal refractive, diffractive, refractive-diffractive, with extended depth-of-field, trifocal, and quadrifocal types, are routinely implanted in the capsular bag. However, because of their specific structure, which may contribute to the occurrence of adverse photic phenomena, surgeons must adhere to quite stringent patient eligibility assessment criteria [5]. This is particularly relevant considering that, in the event of complications (residual refractive defects, intolerance of halo and glare symptoms, impaired contrast sensitivity, or lack of neuroadaptation) replacing the implant poses the risk of serious complications including posterior capsular rupture and retinal detachment, and, ultimately, the risk of failing to meet patient expectations regarding visual quality [18]. Consequently, multifocal implants intended for placement in the ciliary sulcus, referred to as supplemental intraocular lenses (sIOLs), piggyback, or add-on IOLs, emerge as an interesting treatment option for presbyopia. The alternative implantation site of the IOL enables easy removal or replacement when necessary. As a result, it becomes possible to broaden the eligibility criteria, thereby making this treatment modality accessible to a wider population of patients. In light of the considerations above, the subject of this study was to evaluate the efficacy and safety of simultaneous implantation of a monofocal intraocular lens and a piggyback multifocal REVERSO lens in the ciliary sulcus in the surgical treatment of cataract.
MATERIAL AND METHODS
The prospective research project was conducted between November 2014 and January 2020 at the Department of Ophthalmology, Faculty of Medicine, Medical University of Warsaw (Poland). To be considered eligible for study participation, patients had to meet four inclusion criteria, including uncomplicated bilateral cataract, age range between 35 and 85 years, motivation to undergo the proposed method of visual correction, and voluntary consent to participate in the clinical trial. First, patient history was collected, encompassing ophthalmological and general medical conditions, and social background. This was followed by a basic ophthalmic examination and additional preoperative tests including corneal topography, assessment of corneal endothelial cell density, optical coherence tomography (OCT) of the anterior segment, and ocular ultrasound (in cases where fundus examination was difficult). Additionally, macular OCT examination was conducted (when ophthalmoscopy image quality was poor and degenerative changes were suspected) along with biometric evaluation. The exclusion criteria encompassed:
Refractive errors and ocular anatomical characteristics: irregular or regular astigmatism > 1.3 Dcyl; high myopia (ocular axial length > 27.0 mm); high hyperopia (ocular axial length < 20.0 mm); shallow anterior chamber (defined as < 2.2 mm).
Diseases of the posterior pole of the eye: age-related macular degeneration, diabetic and/or hypertensive retinopathy, and glaucoma (relative criterion – only in patients with documented advanced changes in optic nerve disc and visual field defects along with severe dry eye syndrome as a complication of long-term local pharmacotherapy for glaucoma).
Other ophthalmic conditions: weakness of the ligamentous apparatus (e.g. in pseudoexfoliation syndrome, past injury); reduced corneal endothelial cell count (defined as < 1000 cells/mm3) or known corneal dystrophy; changes associated with a history of uveitis, synechiae of the iris; pigment dispersion syndrome leading to glaucoma or elevated intraocular pressure; eyelid abnormalities affecting the quality of vision (ptosis obscuring the visual axis, papillary abnormalities compressing the cornea and inducing variable astigmatism, ectropion); dry eye syndrome (a relative criterion; only when symptoms had a significant adverse effect on daily life and quality of vision).
Ophthalmic surgery: history of corneal refractive procedures.
Social background: patients working in poor lighting conditions; patients who regularly or professionally operate vehicles during night-time hours; patients with potentially unrealistic expectations regarding the proposed treatment, or high visual requirements, lacking adequate motivation and unwilling to accept the compromises associated with the suggested correction method.
Originally, a total of 83 patients (166 eyes) were found to be eligible for study participation. Ultimately, the statistical analysis included 80 patients (160 eyes). Excluded were cases with incomplete data due to missed follow-up appointments on scheduled dates and instances where the patient did not proceed with surgery on the other eye due to health issues (such as newly diagnosed cancer) or personal reasons (patient decision). Eligible patients who provided their voluntary consent and signed a consent form after receiving detailed information about the study, were randomly assigned to either of two groups: the study group, referred to as the Reverso group (GR), or the control group (CG). They subsequently underwent uncomplicated cataract surgery on both eyes, with approximately one-month interval between the procedures. All procedures were performed by a single experienced surgeon. After cataract removal, patients in both groups were implanted with acrylic monofocal posterior chamber IOLs from Alcon (AcrySof IOL, model SA60AT). Patients in the Reverso group were additionally implanted with piggyback multifocal IOLs (REVERSO) from the French manufacturer Cristalens.
REVERSO IOL
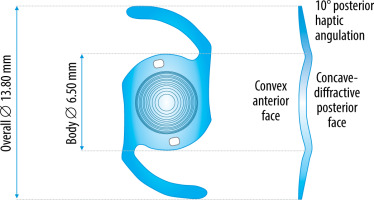
REVERSO, the implant used in the study, is a one-piece, spherical multifocal intraocular lens made of hydrophilic acrylic, designed for implantation in the ciliary sulcus in pseudophakic patients. It has a convex anterior face and concave diffractive posterior face with step apodization of 3.0 mm to 4.5 mm. The optical diameter and total length of the IOL is 6.5 mm and 13.8 mm, respectively. The edges of the optical part around the perimeter and the open-loop haptic parts are rounded, minimizing trauma to surrounding structures (Figure 1).
The standard dioptric power for distance vision is 0.00 D, with +3.00 D addition for near vision. The dioptric power for distance may range from –3.00 Dsph to +3.00 Dsph (in 0.5 increments). The refractive index of the lens is 1.46. Light distribution is conditioned by pupil width, so that the superimposed addition does not impair distance vision. In photopic conditions, light distribution is 50% for distance and 50% for near, while in mesopic conditions it is 65% and 35% for distance and near, respectively, with the aim of minimizing halo effects. Based on the manufacturer’s specifications, theREVERSO lens does not cause any additional spherical aberrations. It may be implanted using a single-use injector with the recommended incision size of 1.8 to 2.2 mm [19].
The study participants were followed up for approximately six months after surgery. Follow-up assessment of individual eyes performed at one and three months postoperatively consisted of automatic refractometry and keratometry, visual acuity testing (using Snellen charts): uncorrected and best corrected for distance and near vision, biomicroscopic evaluation of the anterior and posterior eye segments, and measurement of intraocular pressure. Uncorrected and best corrected distance and near visual acuity was evaluated bilaterally at one and three months after surgery on the second eye. At the six-month follow-up visit, patient assessment consisted of binocular contrast sensitivity testing, spherical aberration testing, flaremetry, and a questionnaire test to determine patient satisfaction with the quality of vision and their reliance on spectacle correction.
Statistical analysis
Statistical analysis was conducted using IBM SPSS software. The normality of distribution was evaluated with the Shapiro-Wilk test (samples with n < 100), while also considering the values of skewness and kurtosis. The difference in quantitative variables for the two groups was analysed with the parametric Student’s t test, assuming normality of distribution. For the variables that did not exhibit a normal distribution according to the Shapiro-Wilk (or Kolomogrov-Smirnov) test, skewness was determined. Where the absolute value of skewness was less than 2 for a variable, a normal distribution approximation was applied, and parametric tests were selected for analysis. When comparing two quantitative variables that did not display a normal distribution and had a skewness of more than 2, non-parametric Mann-Whitney U tests were employed. A significance level of α = 0.05 was adopted for analysis.
Ethical standards
The research project adhered to the principles outlined in the Declaration of Helsinki and received approval from the Bioethics Committee of the Medical University of Warsaw (Approval No. KB/151/2015). Every eligible patient, upon receiving detailed information about the study, signed a voluntary informed consent form to participate.
RESULTS
Preoperative characteristics of study groups
The analysis found that both the Reverso group and the control group were statistically comparable in terms of the sociodemographic characteristics examined (Table I). There were no significant differences in age (p = 0.054) or sex distribution (p = 0.385) between the groups. Furthermore, there were no significant differences in educational background (p = 0.321).
Table I
Demographics of patients in the Reverso group and in the control group
| Parameter | Reverso group | Control group | p |
|---|---|---|---|
| (n=48) | (n=32) | ||
| Age (years) | M=65.4 | M=69.6 | 0.052 |
| Men | 37.5% | 28.1% | 0.385 |
| Women | 62.5% | 71.9% | |
| Higher education | 56.3% | 43.8% | 0.321 |
| Secondary education | 35.4% | 37.5% | |
| Primary education | 8.3% | 18.8% |
The groups were also comparable in terms of preoperative ocular anatomical characteristics (Table II) and concomitant ophthalmic pathologies (Table III).
Table II
Comparison of visual parameters in the Reverso group and in the control group
[i] M – mean; SD – standard deviation; p – probability value; AL – axial length; CECs – number of corneal endothelial cells before surgery; ACD – anterior chamber depth; AST. ORBS. – degree of astigmatism assessed by ORBScan topography; CYL – degree of astigmatism assessed by keratometry; IOP – intraocular pressure; CCT – central corneal thickness
Assessment of treatment efficacy
Visual acuity
There were no significant statistical differences in uncorrected distance visual acuity (UDVA) between the groups (Table IV). In both groups, at three months of follow-up, good monocular UDVA (RG: V = 0.79 vs. CG: V = 0.78) and very good binocular UDVA (RG: V = 0.97 vs. CG: V = 0.92) were achieved. Best corrected distance visual acuity after three months was also comparable in both groups (RG: V = 0.97 vs. CG: V = 0.98) (Table V).
Table IV
Descriptive statistics for uncorrected distance visual acuity (UDVA) in the Reverso group and the control group at different measurement time points
| Visual acuity | Reverso group | Control group | ||||
|---|---|---|---|---|---|---|
| M | Me | SD | M | Me | SD | |
| UDVA 1M | 0.82 | 0.80 | 0.13 | 0.78 | 0.80 | 0.21 |
| UDVA ODS 1M | 0.95 | 1.00 | 0.11 | 0.89 | 0.90 | 0.14 |
| UDVA 3M | 0.79 | 0.80 | 0.16 | 0.78 | 0.80 | 0.23 |
| UDVA ODS 3M | 0.97 | 1.00 | 0.11 | 0.92 | 1.00 | 0.19 |
Table V
Descriptive statistics for best corrected visual acuity (BCVA) for distance in the Reverso group and the control group at different measurement time points
| Visual acuity | Reverso group | Control group | ||||
|---|---|---|---|---|---|---|
| M | Me | SD | M | Me | SD | |
| BCVA 1M | 0.86 | 0.90 | 0.13 | 0.89 | 1.00 | 0.17 |
| BCVA ODS 1M | 0.95 | 1.00 | 0.10 | 0.94 | 1.00 | 0.09 |
| BCVA 3M | 0.93 | 1.00 | 0.11 | 0.92 | 1.00 | 0.11 |
| BCVA ODS 3M | 0.97 | 1.00 | 0.10 | 0.98 | 1.00 | 0.13 |
Best uncorrected near visual acuity was found to be significantly better in the Reverso group compared to the control group at all measurement time points, both monocularly and binocularly (UNVA 3M ODS RG: V = 0.5 vs. CG: V = 1.09). In the Reverso group, the patients could read almost the smallest print size (min. 0.75) one month postoperatively, while after three months, all of them were able to read the smallest print examined (Table VI).
Table VI
Comparison of UNVA in the Reverso group and the control group at different measurement time points
Contrast sensitivity
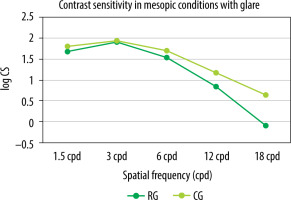
Contrast sensitivity was evaluated using the Functional Vision Analyzer (FVA), based on the Functional Acuity Contrast Test (F.A.C.T.). The test was performed binocularly in distance vision (simulated viewing distance of 6 m) under photopic conditions (85 cd/m2) and separately under mesopic conditions (3 cd/m2) with a glare of 1 lux. A statistically significant difference in contrast sensitivity was observed among patients in the control group compared to the Reverso group for the frequencies of 1.5, 3, 6, and 18 cpd (cycles per degree) under photopic conditions (Figure 4) and for the frequencies of 1.5 and 18 cpd under mesopic conditions (Figure 5). In mesopic conditions, the majority of patients experienced a slight decline in contrast sensitivity across various frequencies, except for 1.5 cpd and 3 cpd, where the results in the Reverso group remained unchanged.
Figure 2
Mean contrast sensitivity in the Reverso group and in the control group examined bilaterally in photopic conditions (85 cd/m2 )
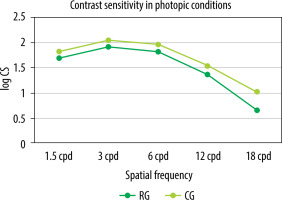
Figure 3
Mean contrast sensitivity in the Reverso group and in the control group examined bilaterally in mesopic conditions (3 cd/m2 ) with glare (1 lux)
Spherical aberrations
Similar levels of aberrations (total HOAs) were detected in both groups (RG: M = 0.16 vs. CG: M = 0.16), Third, Fourth, Trefoil, COMA (RG: M = 0.08 vs. CG: M = 0.07), Tetrafoil, 2nd Astig and Spherical.
Halo and glare
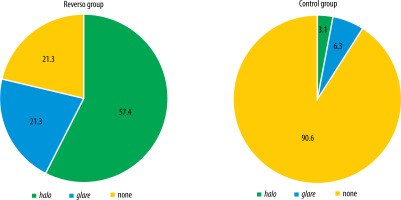
The subjective perception of adverse photic phenomena was shown to be significantly less frequent in the control group (p < 0.001) compared to the Reverso group (no halo and glare observed in the RG: 21.3% vs. CG: 90.6%) (Figure 2). In the Reverso group, the incidence of halo was significantly higher (57.4% vs. 3.1%; p ≤ 0.001). Nevertheless, ultimately, there was no significant difference between the groups in how the perception of adverse photic phenomena influenced the overall subjective quality of vision (p = 0.512). All patients in the control group (100%) reported that the presence of these adverse effects had no impact on their quality of vision, while in the Reverso group, nearly all (95.7%) stated the same.
Patient satisfaction
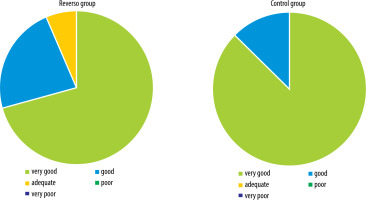
No significant differences were found between the study groups in the subjective satisfaction of patients with the visual outcome of surgery (RG: M = 4.65; Me = 5.0; min. 3.0; max. 5.0; SD 0.6 vs. CG: M = 4.88; Me = 5.0; min. 4.0; max. 5.0; SD 0.34). Most patients rated their quality of vision after cataract surgery as very good (Figure 3).
Examination of the functional quality of vision based on the Visual Function Questionnaire (VF-14), which evaluates 14 vision-related daily activities, in the version without spectacle correction, revealed significantly higher (p ≤ 0.001) results in the Reverso group (RG: M = 95.99; Me = 98.0; min. 75.0; max. 100.0; SD 5.59) compared to the control group (CG: M = 90.3; Me = 91.0; min. 75.0; max. 98.0; SD 5.65). However, no such difference between the groups was observed in questionnaire results in the version with spectacle correction (RG: M = 99.43 vs. CG: M = 99.88; p = 0.349).
Dependence on spectacle correction
In the Reverso group, significantly more patients achieved complete spectacle independence (RG: 91.7% vs. CG: 3.3%). In the control group, incomplete spectacle independence (CG: 33.3% vs. RG: 0%) and no independence of spectacle correction (CG: 62.5% vs. RG: 8.3%) were noted.
Assessment of treatment safety
Tonometry
Significantly higher intraocular pressure was found in the control group (M = 13.82) compared to the Reverso group (M = 13.00) at three months postoperatively. However, in both groups, there was no trend to postoperative rise in intraocular pressure, with average measurements remaining within the lower limit of normal.
Condition of anterior segment of the eye
No statistically significant differences were found between the groups in the occurrence of various anterior segment abnormalities. In most patients, it remained within the range of norm (RG: 66.0% vs. CG: 62.3%). In the remaining cases, fine pigment deposits were observed on the endothelium (RG: 6.8% vs. CG: 8,7%), on the iris (RG: 2.9% vs. CG: 1.4%), on the IOP (RG: 2.9% vs. CG: 5.8%); iris thinning (RG: 3.9% vs. CG: 7.2%); irregular pupil shape (RG: 3.9% vs. CG: 1.4%); mild early posterior capsule opacification (RG: 6.8% vs. CG: 11.6%). In the CG, postoperative corneal erosion with associated edema was noted in one patient. The complication subsequently resolved following standard postoperative treatment.
Flaremetry
No statistically significant differences were found in flaremetry results (p = 0.862) between the Reverso group (RG: M = 7.74; min. 2.1; max. 16.0; SD 3.99) and the control group (CG: M = 7.92; min. 3.4; max. 19.20; SD 3.49). The mean values of the inflammatory marker were within the normal range, i.e. ≤ 10 in both groups.
Duration of surgical procedure
The duration of surgery was significantly longer in patients who were implanted an add-on REVERSO IOL (RG: M = 15.69 min) compared to single IOL implantation (CG: M = 10.15 min).
Complications
No serious complications of treatment were observed. However, non-serious complications were significantly more prevalent (p < 0.001) in the Reverso group (RG: 30.3% vs. CG: 4.8%). There were five cases of elevated intraocular pressure on the first postoperative day, all successfully managed with conservative treatment (two in RG, three in CG). In addition, there was one case of increased inflammation with pupillary fibrin membrane, which resolved after intravenous steroid treatment (one in RG), and 11 cases of REVERSO IOL decentration (with no effect on the final quality of vision) and one case of recurrent IOL dislocation in front of the iris.
DISCUSSION
Based on the findings presented, implantation of a supplemental multifocal REVERSO lens during cataract surgery can be regarded as a safe and satisfactory method for both the patient and the surgeon. The proposed solution resulted in very good binocular uncorrected visual acuity and high levels of subjective patient satisfaction confirmed by very good VF-14 questionnaire results, which allows a reliable assessment of the visual functioning of patients on a daily basis. In the literature, the application of the REVERSO lens has thus far been documented exclusively by French researchers, led by the ophthalmologist Myriam Cassagne. In their study, the authors observed slightly weaker results at one month compared to those reported in this article for binocular UDVA (0.03 logMAR), followed by slightly better results at one-year follow-up (0.011 logMAR). Furthermore, BCVA for distance at one month (0.014 logMAR) was slightly superior to our results at one-month follow-up (0.03 logMAR), but comparable to our observations at three months (0.013 logMAR), with an additional improvement in BCVA achieved at one-year follow-up, reaching 0.008 logMAR [20]. In our study, most patients achieved very good binocular uncorrected near visual acuity at one-month follow-up. After three months, the results were excellent, with all patients being able to read the smallest print on the Snellen chart. Similar results were reported by Cassagne et al., who recorded the UNVA of 0.12 logMAR and 0.14 logMAR at one month and one year of follow-up, respectively. Reviewing the results obtained with other multifocal sIOLs, Gerten investigated the add-on multifocal IOL MS 714 PB from HumanOptics AG, achieving the UDVA of 0.02 logMAR and UNVA of 0.08 logMAR at three-month follow-up [21]. Schrecker achieved slightly better UDVA (0.00 logMAR) and UNVA (0.10 logMAR) levels at one-year follow-up after implantation of the add-on IOL Diff-sPB (HumanOptics AG, Germany) [22]. In 2020, Palomino-Bautista studied the application of the trifocal IOL 1stQ AddOn (Medicontur) implanted in the sulcus in pseudophakic eye, achieving good results at six-month follow-up, with the UDVA of 0.03 logMAR and UNVA of 0.12 logMAR [23]. Clinical experience shows that time and patient motivation play an important role in the process of adaptation to new optical conditions. Considering that the literature reports usually concern multifocal sIOLs implanted in a secondary procedure, with the placement of an add-on IOL in the sulcus of a previously pseudophakic eye, it is important to recognize that outcomes achieved in a simultaneous procedure may be slightly less robust. This is due to the additional possibility of correcting the residual refractive error remaining after the first surgery during a secondary procedure. Contrast sensitivity outcomes observed in patients treated with the REVERSO lens were significantly lower compared to those determined in the study’s control group, which is a characteristic feature of multifocal IOLs. Interestingly, poorer contrast sensitivity did not result in lower satisfaction with the quality of vision or functional vision, as assessed by questionnaire. It is important to note that as many as 91.7% of patients achieved complete spectacle independence, which confirms the efficacy of planned treatment. However, a direct comparison of contrast sensitivity outcomes reported in the literature is challenging due to inconsistencies in the methods used for examination. Currently, the CSV-1000, FVA, or Optec 6500 Vision Tester are the most popular testing instruments used in patient assessment [7, 24, 25]. However, the techniques available for conducting examinations are highly variable (simulations of distance and close-up vision, monocular and binocular assessment, evaluation under photopic and mesopic conditions with varying glare intensities) and arbitrarily selected, so comparing them across the studies is essentially unfeasible. In future clinical practice, it is important to establish a standardized contrast sensitivity testing protocol. This will enable better validation of results and facilitate comparisons with findings from other researchers. When examining the occurrence of undesirable spherical aberrations and their subjective perception among patients with the REVERSO IOLs, a significant majority (95.7%) reported that the presence of halo and glare had no impact on their subjectively evaluated quality of vision. This outcome is satisfactory, especially considering that the presence of halo and glare can significantly reduce the level of patient satisfaction with visual quality. Verdonck et al. presented the outcomes of Sulcoflex IOL implantation at a five-year follow-up, with 80% of patients reporting adverse photic phenomena (with coexisting pigment deposits in the anterior eye) that led to uncomplicated removal of the add-on lenses in 41.94% of study participants [26]. Even though removal is not the originally planned stage of treatment, the possibility to safely explant the IOL from the sulcus in order to replace it or remove it altogether seems to be an important advantage in the treatment of presbyopia compared to the use of IOLs implanted in the capsular bag. The authors of this study found no persistent pathological alterations in the patients’ intraocular pressure or chronically elevated inflammation. The minor complications observed had no adverse effect on the quality of vision, leading to a favorable assessment of the treatment by the study participants. Even though decentralization of supplemental IOLs resulting in impaired vision has been reported in the literature [7], we had no such experience in our study. The slightly longer surgery time is likely attributed to a learning curve; however, ultimately, the technique of supplemental IOL implantation in the sulcus is described as straightforward.
CONCLUSIONS
The results of the six-month follow-up of patients treated by REVERSO lens implantation, as presented above, show that the procedure is effective and safe for both patients and operators. The potential reversibility of the treatment by removing the REVERSO implant, if necessary, represents a valuable advantage over multifocal lenses implanted in the capsular bag. Furthermore, the surgical technique, which remains within the basic skills of anterior eye surgeons, contributes to the growing interest in IOLs placed in the ciliary sulcus, and their increased use.

 ENGLISH
ENGLISH