Summary
The manuscript addresses a crucial clinical issue concerning radiation exposure, which is essential not only for patients but also for healthcare providers. In recent years, there has been increasing emphasis on minimizing radiation exposure. Our study demonstrates the feasibility of performing left-sided ventricular arrhythmia ablation using advanced technology (electroanatomic mapping systems) in combination with an appropriate approach by the cardiologist. This finding highlights the potential to eliminate radiation exposure while maintaining effective invasive treatment for left-sided ventricular arrhythmias.
Introduction
Catheter ablation (CA) is the gold standard for the treatment of patients with symptomatic, idiopathic ventricular arrhythmias (VAs). These VAs can manifest as premature ventricular contractions (PVCs) or ventricular tachycardia (VT), which can be non-sustained or sustained [1]. Using a three-dimensional electroanatomic mapping (3D EAM) system makes it possible to generate precise cardiac anatomy, map the activation of arrhythmia origins, and precisely navigate catheters. This technology not only reduces but also potentially eliminates radiation exposure completely [2, 3]. However, additional navigation, including fluoroscopy, may be beneficial for verifying or securing the catheter position in challenging scenarios, particularly in proximity to the coronary arteries. Regrettably, this approach is associated with exposure to ionizing radiation. The effectiveness of CA varies depending on the location site of VAs. If VAs originate from the right ventricular outflow tract (RVOT), the success rate of CA exceeds 85% [4, 5]. However, due to the thicker myocardium of the LV, the source of arrhythmia may be located deep within the myocardium. Thus, the effectiveness of endocardial CA can be reduced [6]. What is challenging is that left-sided arrhythmias are more likely to be located in epicardial sites [7]. When the arrhythmia originates epicardially, precise localization and ablation of the arrhythmogenic site can be challenging with an endocardial approach [8]. Moreover, left-sided VAs’ CA is often challenging due to the anatomical proximity to the coronary arteries, especially in the epicardial approach [9]. Furthermore, in left-sided VAs, the arrhythmogenic site may be located near the conduction system, which makes it difficult to deliver sufficient power to eliminate the arrhythmogenic site without causing a conduction block [10].
While CA of left-sided VAs is generally considered safe, there is still a risk of encountering minor or major complications. Minor complications may include small pericardial effusion and vascular access issues such as groin hematoma, pseudoaneurysm, or arterio-venous fistula. On the other hand, major complications, although less common, can include more severe outcomes such as pericardial tamponade, atrioventricular (AV) block, left bundle branch block (LBBB), significant bleeding, thromboembolic events, myocardial infarction, or aortic dissection.
Limited data exist about the feasibility, efficacy, and safety of the zero-fluoroscopy approach in patients with idiopathic VAs from left-sided locations.
Aim
The study assessed the feasibility, efficacy, and safety of performing CA using a zero-fluoroscopy approach in patients with left-sided idiopathic VAs with the 3D EAM system.
Material and methods
Study population, design, and patient selection
This observational, retrospective, and single-center experience study recruited patients with left-sided, idiopathic VAs who had undergone elective CA at our center. Fifty-three consecutive patients from June 2020 to December 2023 were enrolled in this study.
Patient selection for CA followed established guidelines [11]. Inclusion criteria for this study were age over 18 years and LV ejection fraction (LVEF) above 40%. Exclusion criteria were structural heart disease with a history of previous myocardial infarction or prior revascularization procedures, and significant (moderate or severe) valvular disease, significant (moderate or severe) chest wall deformities, and implanted pacemaker systems. Patients presenting with sustained VT, with an active inflammatory state, suspected myocarditis, or suspected channelopathies on the ECG were not included in this study. Patients with a high initial probability of epicardial origin based on PVC morphology in the ECG were excluded, as this would inherently require the use of fluoroscopy. Transthoracic echocardiography was conducted in all patients, both before and after the CA procedure. Some patients had previously undergone imaging studies such as cardiac computed tomography scans, coronary angiography, or cardiac magnetic resonance imaging during outpatient visits or previous hospitalizations, which served as references for operators and confirmed the idiopathic nature of the arrhythmia.
Since the inception of the center, the ALARA (as low as reasonably achievable) principle has been employed for CA procedures, emphasizing the reduction of radiation exposure. All left-sided VA CA procedures were considered for the analysis, resulting in a total of 53 patients after applying the exclusion criteria.
Procedure characteristics
In all patients, transaortic access was obtained via puncture of the femoral artery, followed by the insertion of a single 8F vascular sheath. The bi-directional irrigated tip radiofrequency (RF) ablation catheters (Thermocool SmartTouch SF), the 3D EAM system (Carto, Biosense Webster, Irvine, California, USA) and the Smartablate RF generator were used for all procedures. For safety reasons, the operators and EP staff wore lead aprons during the procedure in case conversion to a fluoroscopy-guided procedure became necessary. All procedures were performed by two operators with over 15 and 30 years of experience in invasive cardiac electrophysiology.
The catheters’ contact force sensors were calibrated outside the patient to safely introduce them through the arterial system without using fluoroscopy. In cases of persisting resistance (in the initial segment of the artery), access from the femoral artery on the opposite side was considered. In cases of persisting resistance (further from the puncture site), fluoroscopy with the assistance of a hydrophilic guidewire and an 8F long sheath (SL0; Abbott) or steerable sheath visible in the 3D EAM system (VIZIGO) was considered.
We developed our protocol for performing zero-fluoroscopy left-sided VA CA, which relies on creating a fast anatomical map (FAM) to visualize the ascending aorta and arch of the aorta. In cases where there was suspicion of an arrhythmia site located within the aorta, we also utilized local activation time (LAT) mapping. For the procedure’s safety, we marked the location of the coronary artery ostia (when the catheter inadvertently entered the artery’s ostium) and aortic valves on the FAM. In cases where arrhythmia sites were located beneath the aortic valve, the operator crossed the aortic valve using a J-shaped electrode curve and started LAT mapping of the LV. If the arrhythmia was infrequent during the procedure, an isoproterenol infusion and/or digoxin injection were used. If the problem persisted, the pace-mapping method was used to target and confirm the RF application site. During an arrhythmia, the site of earliest activation was located, and served as the target for the RF application. During application, power outputs ranged from 30 W to 45 W, depending on the location of the arrhythmia and contact force, with a temperature not exceeding 48°C.
The procedural duration was defined as the time interval from the arterial puncture to the removal of arterial sheaths at the end of the procedure. The Smartablate RF generator automatically recorded the ablation time and the number of applications.
Acute procedural success was defined as the absence of targeted arrhythmia recurrence within 15 min after the last RF application. Long-term efficacy was defined as a significant reduction in arrhythmia burden (below 1,000 PVCs and absence of any VT) observed in repeated 24-hour Holter monitoring. The minimum follow-up period was 6 months.
Minor complications included pericardial effusion and vascular access issues (groin hematoma, pseudoaneurysm, or arterio-venous fistula). Major complications included pericardial tamponade, AV block, significant arterial bleeding, thromboembolic events, myocardial infarction, or aortic dissection.
Statistical analysis
Statistical analysis was conducted using Statistica software version 13.3. Categorical variables are expressed as numbers and percentages and were compared using the χ2 test. Continuous variables are presented as mean (standard deviation, SD) if the distribution is normal and median (interquartile range, Q1–Q3) if the distribution is non-normal. The comparison of variables between groups was performed using the t-test for variables with a normal distribution and the Mann-Whitney U test for non-normally distributed variables. Statistical significance was considered at p < 0.05.
Results
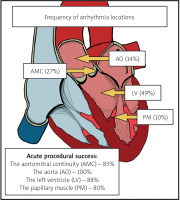
Baseline characteristics are shown in Table I. Of the 53 patients, 24 (45%) were male. The mean age was 55.8 ±14.9 years. Patients’ mean left ventricular ejection fraction (LVEF) was 57 ±6%. Of 53 patients, 11 (21%) presented PVCs with non-sustained VT (nsVT), and 42 (79%) exhibited PVCs without nsVT. The exact locations of the origin of arrhythmia are shown in Figure 1.
Table I
Baseline characteristics
CA was performed without fluoroscopy in 44 (83%) cases. The use of fluoroscopy was necessary when the origin of the arrhythmia was located near the coronary artery (which required coronary angiography), which occurred in 4 (7.55%) cases. The challenges in passing a catheter through the arterial system, which necessitated the use of fluoroscopy, accounted for the 5 (9.43%) cases. The average procedural duration was 97.8 43.2 min, with a mean application time of 8.1 ±8.1 min and a median number of applications of 4 (Q1–Q3: 2–11). The duration of zero-fluoroscopy procedures was 93.04 ±39.91 min, with an application time of 7.03 ±7.48 min. The median number of applications during zero-fluoroscopy procedures was 4 (Q1–Q3: 2–9). In the fluoroscopy group, the average fluoroscopy time was 2.2 ±3.1 min, and the radiation dose was 68.6 ±141.9 mGy.
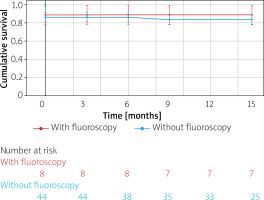
The mean follow-up duration was 12.06 ±4.61 months. The Kaplan-Meier curve illustrates the event-free survival rate from VAs in groups with and without fluoroscopy (Figure 2), with 7 out of 44 patients experiencing long-term recurrences in the zero-fluoroscopy group and 1 out of 9 patients in the fluoroscopy group.
In most unsuccessful procedures (5 out of 8 cases), it was suspected that the arrhythmia originated from an epicardial or deep intramural location, which was beyond the reach of endocardial CA. In this group, we usually observed repetitive transient effects of application, and we could not achieve ideal pace-mapping. One of these patients underwent a successful repeat procedure via coronary sinus access 7 months later and has remained arrhythmia-free during follow-up. Two patients have an arrhythmia burden below 5000 PVCs per day on 24-hour Holter monitoring, so no further re-ablation procedures were performed. Two patients declined to undergo another procedure. The remaining 3 patients, who did not have suspected epicardial or deep intramural locations, are awaiting another ablation procedure.
The unsuccessful procedures were more prolonged (119.25 ±35.00 vs. 96.71 ±44.00 min) (p = 0.102). In these patients, more RF applications were performed, with a median of 9.5 (Q1–Q3: 4–16) vs. 4 (Q1–Q3: 2–10) (p = 0.170), and the duration of these applications was longer (10.95 ±12.70 vs. 7.94 vs 7.69 min) (p = 0.862).
In 2 (3.7%) patients, a minor complication of groin hematoma was observed. Major complications were not observed.
Discussion
The current study presents the feasibility, techniques, and results of CA procedures for various left-sided VAs without fluoroscopy. Cardiologists could perform most procedures without fluoroscopy; the operator’s proficiency and mindset are the principal limitations. A comprehensive understanding of cardiac anatomy remains paramount. Thus, this approach should be incorporated into all electrophysiology training programs to minimize radiation exposure and mitigate the risk of late radiation complications.
Conflicting data exist regarding the feasibility of performing VA CA regardless of the arrhythmia location without fluoroscopy because it mainly depends on the center and the operator.
Some researchers demonstrate in their studies the effectiveness of procedures without fluoroscopy and do not include fluoroscopy-based procedures in their analysis, making it impossible to assess the feasibility. In the study by Filippo Lamberti et al., the authors found that idiopathic VT ablation without fluoroscopy was safe and effective in nineteen patients. They used a combination of EAM and ICE. Notably, none of the patients required a transseptal puncture or an epicardial approach. The acute success rate was 100%, with no complications documented in any patients. VT recurrences were observed in 2 (11%) patients [12]. In the analysis of fluoroless CA, Mansour Razminia et al. reported 30 CAs for PVC and 14 CAs for VT. Acute procedural success for PVC ablations was achieved in 28 (93.3%) patients, with recurrences occurring in 2 (6.7%) patients. For VT ablations, the acute procedural success rate was 100%. However, the VT group had 3 (21.4%) recurrences. In both studies mentioned above, the study groups were relatively small, and the necessity of using fluoroscopy rates was not specified [13].
However, the percentage of these procedures performed without fluoroscopy has been increasing in recent years. Some publications indicate that VA ablations without fluoroscopy are associated with a rapid learning curve, which tends to plateau after about 15 procedures [14]. Only some studies demonstrate the feasibility and effectiveness of performing ablation of left-sided VAs via an aortic approach without the use of fluoroscopy. In the study by Karkowski et al., the authors found that left-sided VAs CA could be performed without fluoroscopy in 21 out of 43 patients (49%) [15]. In comparison, in our study, the rate was 83%. However, it is important to note that this study included procedures conducted between 2014 and 2018; these results are notable for that period. During this period, in other centers and publications, VA ablations were performed with nearly 100% reliance on fluoroscopy. It was only after 2019 that the approach to using fluoroscopy and performing fluoroless procedures began to change significantly [16].
Fluoroscopy may be necessary when the catheter encounters difficulty passing the arterial system. It is often due to disseminated atherosclerosis in the iliac and femoral arteries, and aorta. When resistance is encountered during catheter passage, accessing the femoral artery from the opposite side (if resistance occurs in the initial segment of the artery) or using fluoroscopy should be considered to ensure procedural safety. An alternative approach to address this challenge could involve using a hydrophilic guidewire and a long vascular sheath, especially a steerable one. However, this solution also requires the use of fluoroscopy. Passing through the aortic valve poses another potential challenge, especially when encountering resistance at this location. In such cases, fluoroscopy may be necessary to ensure the safe crossing of the catheter through the aortic valve and avoid damage to the coronary arteries. The catheter must be appropriately curved in the aortic arch and passed through the valve using a loop technique. Fluoroscopy may also be employed when the arrhythmia site is in proximity to a coronary artery, necessitating coronary angiography. RF CA is contraindicated if the arrhythmia location is within 1 cm of a coronary artery. In such cases, RF CA may induce coronary vessel spasm, thrombosis, or mechanical damage of arteries. The specific arrhythmia location, such as those in the papillary muscle, can also pose a challenge due to catheter instability.
In our view, the most significant limitation affecting the success of the endocardial CA procedure is the epicardial or deep intramural location of the arrhythmia, not the use of fluoroscopy. Zero-fluoroscopy CA procedures are feasible within the LV or the aorta but have a few limitations, as described above.
In recent years, ICE has become more prevalent in facilitating CA for VAs [12, 17]. ICE enables the intraprocedural identification of myocardial substrates, optimization of catheter-tissue contacts, recognition of anatomical obstacles to ablation, and early detection of complications. Additionally, studies have shown that ICE allows for effective CA of left-sided VAs without the use of fluoroscopy, even in the aorta near the coronary ostia [18–20]. However, other authors suggest that zero-fluoroscopy CA can be successfully performed in almost all patients with VAs originating from the aorta by operators with prior experience in near zero-fluoroscopy techniques and after appropriate training [21]. Furthermore, the cost of the ICE catheter can be a barrier to adopting this technology during the standard procedures in some centers and countries. Additionally, the use of ICE requires extra-venous access, which slightly raises the risk of vascular access complications.
The increasingly advanced 3D EAM systems provide more information to the cardiologist during the procedure, enhancing its safety and efficacy. This is confirmed in meta-analyses and multicenter studies [22, 23]. Furthermore, irrigated catheter technology and contact force measurement enable safe and effective procedure execution.
Considering the conservative estimate of an average of 20 min of fluoroscopy time for VA CA procedures, which is even lower than the times commonly reported in the literature [16, 24, 25], we have saved almost 15 h of continuous fluoroscopy time overall. This is beneficial not only for patients but also for all healthcare providers during procedures.