Purpose
Mycosis fungoides (MF) accounts for almost 50% of primary T-cell cutaneous lymphoma (CTCL) cases, and typically starts with polymorphic patches, which eventually progress to plaques and tumors [1]. Histopathologically, MF is hard to distinguish from other inflammatory dermatoses, and classical immuno-histochemical finding shows a CD4 : CD8 ratio greater than 5 [2]. Early-stage MF treatment commonly involves skin-directed therapies (SDT), such as topical corticosteroids, phototherapy, and radiotherapy [3]. In the presented case, superficial brachytherapy using 3D technique was employed, a rarely chosen treatment option for MF. The therapy resulted in complete remission of all facial neoplastic lesions, minimal acute toxicity, and restoration of previous face appearance.
Case description
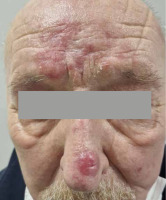
A 74-year-old male patient was admitted to Dermatology Clinic at the University Clinical Centre in Gdańsk, due to erythematous skin patches on the left upper eyelid and forehead. Lesions persisted for six months, and were causing significant pruritus. Despite previously dermatologist-prescribed prednisolone (10 mg daily), the patches continued to grow. Physical examination and peripheral blood results were within normal ranges. Initial diagnostics included cutaneous sarcoidosis, psoriasis, or skin tuberculosis. A biopsy with immuno-histochemical staining revealed a rich T-lymphocyte (CD4, CD8) infiltration of the epidermis with parakeratosis. Absence of tuberculosis schemes dismissed the possibility of sarcoidosis and tuberculosis, leading to a primary diagnosis of pseudo-lymphoma. Previously, the patient was successfully treated with topical corticosteroids. However, after five months, a significant aggravation was observed with the emergence of new lesions on the nose (Figure 1). Therefore, a final diagnosis of MF was made based on new biopsy results. After ruling out systemic involvement, the focus was on a preferred treatment choice. Initially, teleradiotherapy was chosen, but due to proximity of the lens with concomitant early-stage cataracts, 3D brachytherapy was selected as an alternative.
Material and methods
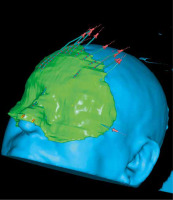
A 3D simulation before mold preparation was performed for optimal dosimetry. For this reason, previously obtained scans for diagnostic purposes were used. The best configuration was found using 12 flexible needles placed 5-8 mm apart from each other in three different groups (Figure 2). In the Department, a mold mask of the patient’s face imprint was manually prepared using a thermoplastic material (hydroxypropyl methylcellulose). A 2-4 mm thick base was applied over the lesions with an additional margin for better adjustment and immobilization. Once the mass dried and solidified, 12 flexible applicators were embedded into the mold to maintain the required 3-5 mm space from the skin. Following this step, the patient underwent a CT scan with the mold in place and a radiopaque marker surrounding the facial lesions under it. This allowed for visualization and re-adjustment of the applicators to ensure adequate coverage of the lesions. An optimized treatment plan was formulated based on the acquired scans, with 1 mm thickness.
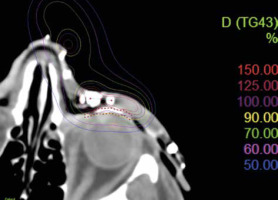
The aim was to irradiate the lesions with a therapeutic dose while minimizing the dose applied to the lens. Total dose was calculated using Oncentra treatment planning system (Electa®). Planning process was conducted entirely in 3D, in accordance with planning target volume (PTV). PTV encompassed macroscopic and radiological areas of tumor infiltration, with an additional margin of 10 mm in the skin plane and 5 mm in depth to ensure better coverage of the lesions. Treatment consisted of 10 Gy in 5 fractions (one fraction per day), targeting the forehead, nose, and upper eyelid. The lens received a mean dose of 5 Gy and fell in a 45-55% isodose range (Figure 3). Treatment was performed with high-dose-rate (HDR) brachytherapy using an iridium-192 (192Ir) source, and afterloading was completed with a Flexitron device. Regression was observed three weeks after the completion of treatment.
Results
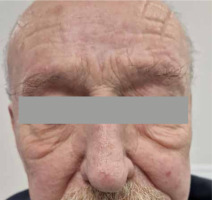
According to CTCAE v. 4.0 scale [4], acute toxicity was estimated as grade 1. A 12-month follow-up showed sustained remission without any late toxicity or recurrence of MF skin lesions on the face (Figure 4). Currently, the patient is undergoing treatment for minor skin patches on the trunk, using HDR brachytherapy with a Freiburg flap applicator. He remains under the supervision of both Dermatology and Oncology Departments.
Discussion
Depending on the stage of MF (i.e., I-IV stage), a treatment is selected. Patients with skin-limited disease are treated with skin-direct therapies (SDTs) [5, 6]. As MF mainly impacts superficial layers of the skin, topical corticosteroids (CS) are usually the first choice of treatment due to their accessibility, low cost, and effective barrier to disease progression. Nevertheless, the evidence supporting steroid therapy is limited, and its effectiveness varies significantly, ranging from 12% to 82%, depending on the disease stage [7]. One should also remember that 10-20% of patients may develop irritant dermatitis, purpura, and rarely systemic outcomes [8]. When steroid resistance occurs, phototherapy is an alternative. Despite effective lesion management, acute side effects, such as erythema (100%), pruritus (25%), and nausea (12%) are constantly reported. Moreover, cumulative doses of PUVA therapy (over 200 treatments) are linked to a 10-30 times higher risk of squamous cell carcinoma [9]. Another option is radiotherapy, where total skin electron beam therapy (TSEB) is the preferred treatment choice. TSEB minimizes skin penetration and simultaneously reduces internal organs toxicity. However, it also effects healthy parts of the skin, which, in the worst scenario, may lead to the loss of its regeneration potential and even the development of new cancer [5]. Still, the use of electrons on irregular surfaces, such as the face is limited. Alternatively, an X-ray beam may be used, but is associated with a higher risk of deeper penetration and healthy tissue damage [10].
The above concerns were the main issues while choosing the treatment for our patient, since not only was the lesion placed on an irregularly-shaped eyelid, but it was also located near the lens. Choosing HDR superficial brachytherapy allowed for a balance between achieving an acceptable dose penetration (5 mm in depth) and limiting the dose on fragile organs, such as the lens. We took advantage of the manually prepared mold to overcome dose variation resulting from anatomical curves. The distance from the skin of applicators ranged from 3 to 5 mm due to the irregular surface. However, new findings suggest that irregularities of applicator distance placement in the same mold, if properly planned, can lead to improved dose distribution and therefore, better CTV coverage without increasing hot spots [11].
Superficial brachytherapy has already been successfully implemented in the treatment of keratinocyte carcinoma. According to the available data, this method’s 5-year relapse-free survival rate is around 94%, with only slight acute toxicity [12-14]. Moreover, both European and American guidelines support its effectiveness and safety, recommending brachytherapy as the first-choice treatment for certain groups of patients, such as the elderly or cases with facial lesions. However, the available data indicate that this method is most effective in lesions with penetration no deeper than 5 mm [15, 16]. Nowadays, there are no direct recommendations provided by either GEC-ESTRO ACROP or EORTC for brachytherapy in mycosis fungoides or other T-cell lymphomas [5, 15]. However, some examples of brachytherapy applications for MF exist in the available literature, presented by case reports [17, 18] and more extended trials [19]. The outcomes were satisfying, with low or no early toxicity. Another work shows brachytherapy as an effective tool for cutaneous lymphomas, especially in elderly patients [20]. To date, our patient remain lesion-free in facial areas, with no signs of late toxicity during regular examinations over 12 months. By choosing brachytherapy, it is possible to balance the pros and cons for patients with early-stage MF, who have a long-life expectancy, are on immunosuppression, or already have a high-rate of photodamage. These groups may benefit from brachytherapy due to its low toxicity, high efficacy in managing lesions and pruritus as well as excellent cosmetic effects, which are crucial given the social burden of the disease. Furthermore, a 3D brachytherapy technique allows for precise estimation of the applied dose, limiting unwanted damage to critical organs and healthy tissues.
In the presented case, the lesion was partially located on the upper eyelid. It should be mentioned that this area is highly heterogeneous due to its varying tissue densities and curvatures. This may result in low accuracy in calculations of absorbed radiation dose, especially when it is based on standard algorithms, such as TG-43. Nevertheless, implementing modern solutions based on the TG-186 algorithm can improve treatment quality and safety [21]. Brachytherapy seems to be a safe and effective treatment. However, long-term toxicity is always a concern. Due to insufficient data, this method is mainly advised for elderly patients to improve local control of minor lesions [15]. Further studies are needed, as this treatment option seems to be a very convincing alternative due to its low toxicity and high efficacy.