Purpose
Uveal melanoma is the most common primary malignant intraocular tumor in adults, with an annual age-adjusted incidence of 5.1 per million cases [1]. It used to be treated by enucleation, but since the Collaborative Ocular Melanoma Study (COMS) publication, plaque brachytherapy is assumed to play an important role in the treatment of posterior uveal melanoma [2, 3]. According to the COMS classification system, brachytherapy was indicated in the following three conditions: small melanomas, with a documented tendency to grow or with clear signs of activity; all medium-sized melanomas; and some large melanomas, with a reasonable potential for preserving vision upon patient consent [4, 5]. Although this procedure is effective, it can lead to various ocular complications [6-8], and often results in significant loss of visual acuity (VA) because of high radiation dose. There are several reasons for this, but the main side effects include retinopathy, maculopathy, cataract, neovascular glaucoma, and nerve atrophy. The severity depends primarily on the amount of incidental irradiation to various tissues and ocular structures that are radiosensitive [9].
Duration of treatment does not seem to be particularly important in the occurrence of late toxicity related to radiobiological dose [10, 11]. However, in a recent study by Miguel et al., statistically significant variables for visual loss were found in a multivariate model, such as apical height, plaque size, juxtapapillary location, and dose to foveola [12]. Multivariate studies conducted by various authors revealed significant values in many different characteristics depending on variables analyzed, including larger size [13, 14], lesser distance to fovea or macula [13, 15], lesser distance to optic nerve [6, 16], dose to optic nerve [14, 17], dose to sclera [14], dose to macula [14], high macular dose rates [18], older age [13], younger age [17], initial VA [17], retinal invasion before treatment [19], tumor shape [20], plaque shape [20], diabetes mellitus [21], and serous macular detachment [22]. Numerous studies have shown that lower irradiation doses were correlated with lower rates of visual loss [23].
The aim of the present study was to analyze the course of VA in patients treated with 125I brachytherapy based on pre-treatment VA evaluation.
Material and methods
Patients diagnosis, treatment, workflow, and treatment features
Patients prospectively and consecutively treated with 125I (ROPES [24] and COMS [25]) plaques for uveal melanoma in the Intraocular Tumors Unit of Intraocular Tumors Unit Intraocular Tumors Unit, Valladolid University Hospital, Valladolid, Spain from January 1, 1996, to May 1, 2022 were included in this study. Patients treated with transpupillary thermotherapy before brachytherapy were excluded.
All patients were initially examined by an ophthalmologist experienced in ocular oncology, and diagnosed with choroidal melanoma. Brachytherapy was performed according to standard protocol of the American Brachytherapy Society (ABS) guidelines [26-28]. The ophthalmologist and oncologist outlined the target, and plaque size was chosen to include the basal margin. The radiation oncologist defined clinical target volume (CTV) considering tumor thickness from B-scan sonography images and safety margin extension of 1-2 mm in all directions. Planning target volume (PTV) could be added by the radiation oncologist in case of a doubt regarding plaque localization or tumor delineation [29].
Applicators were sutured to the sclera and removed after an appropriate time. Tumors were located by transillumination and indirect ophthalmoscopy. Prescribed dose to the tumor apex was 85 Gy in all cases. Plaque heterogeneity correction functions were incorporated in treatment planning. Moreover, collimation of dose through the lip on the gold alloy base and global attenuation factor accounting for the effect of plaque seed in the eye were applied. All patients signed an informed consent form, after being duly informed about possible side effects. Before treatment, the following information were obtained: treatment duration, plaque size, number of seeds (in case of iodine plaques), total activity, and distribution of 125I seeds required to apply the prescribed dose to target volume.
Regular follow-up visits were performed at 1, 3, 6, and 12 months, every 6 months from 1 to 5 years after therapy, and annually thereafter, if local control had been achieved. In practice, the number of revisions may be higher in many patients during the first 5 years, mainly because of special surveillance, and follow-up times may also vary because of hospital stay scheduling. All patients underwent a complete ophthalmologic examination, including Snellen VA measurements.
Visual acuity definition and study groups
Visual acuity is defined as a reciprocal of ratio between the letter size a patient can evaluate and the size a standard eye can recognize. Pre-operatively and post-operatively, VA was recorded in decimal logarithmic scale (V). Linear scales are not meant for clinical records, but they are required for statistical purposes They convert the progression of V values into a linear one, based on Weber-Fechner law stating that proportional increases in stimulus lead to linear increases in perception. One of the most used scales is a VA score (VAS) that relates to V as follows [30]: VAS = 100 + 50 log V. This score is more intuitive because it indicates higher values. On this scale, the value of 100 (V = 1) corresponds to normal vision, while the value of 50 (V = 0.1) represents the limit of legal blindness in our country.
To determine how VA changes over time as a function of pre-treatment VA, the total cohort was divided into 4 groups: (1) Patients who previously had an initial VA of V ≤ 0.1 (VAS ≤ 50); (2) Patients who could see but had a low to moderate VA, ranging 0.1 < V ≤ 0.4 (50 < VAS ≤ 80.1); (3) Patients with medium-high VA, ranging 0.4 < V ≤ 0.8 (80.1 < VAS ≤ 95); and (4) Patients with a very high VA that in this study was considered as V > 0.8 (VAS > 95).
Each of the four groups was studied separately over a 60-month period time to determine the percentage of patients with VA improvement, worsening, or remaining the same VA status. Each semester, in which VA was monitored, was compared with baseline VA values before brachytherapy. Based on our internal standards, patients were classified as having experienced VA improvement when their visual analog scale (VAS) score increased by 10%. Patients were categorized as having suffered a loss of visual acuity when their VA decreased by 10%. If a patient had more than two follow-up visits in the same semester, VA was set as a geometric mean of individual corrections in the linear scale for that semester. For enucleated patients, VA was classified as no light perception at the time of enucleation. Finally, visual outcomes over time were estimated with 95% confidence interval (CI) using Kaplan-Meier analysis, and VA maintenance rates were reported at 3, 5, 10, 15, and 20 years of follow-up. Kaplan-Meier analysis and estimation of differences with log-rank test were performed for the four groups to determine statistically significantly differences.
Results
Patients
From 1996 to June 2022, 305 cases of choroidal melanoma were treated with ophthalmic brachytherapy. A total of 3,618 post-treatment visual follow-up measurements were used in this study, with a median follow-up of 78.2 months (range, 6-254 months) and loss of follow-up of less than 1%. Thirty-one patients underwent an enucleation after brachytherapy. Tables 1 and 2 display baseline patient demographics, tumor characteristics, and doses to the tumor apex for each sub-cohort.
Table 1
Patient and tumor summary statistics for 305 eligible cases. Quantitative variables
Table 2
Patient and tumor summary statistics for 305 eligible cases. Qualitative variables
Sub-cohort 1. initially blind patients, v ≤ 0.1. blind
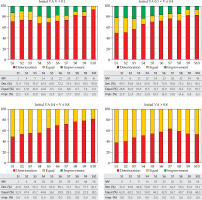
Sixty-one of the 305 patients were included in this sub-group (20% of the total cohort). The preservation of the organ was achieved in majority of patients (82%), and 11 of 61 patients were enucleated. Figure 1 shows how the percentage of patients with lost VA was greater than those whose VA maintained or even improved. A small number of patients gained VA after the intervention. However, this fact did not mean the recovery of vision even in those with a VA close to the limit. In 3 of 57 patients, the vision recovered but after one year, only one patient remained vision with V > 0.1; the rest remained blind. Despite this, a small number of patients reduced over time have gained VA, with values below the limit of 0.1.
Fig. 1
Progression of visual acuity in patients as a function of their initial visual acuity. S1-S10 are the semesters after brachytherapy, and MV show the missing value
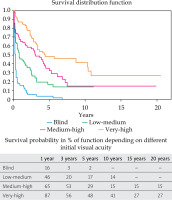
Actuarial Kaplan-Meier curves are described in Figure 2. Visual acuity preservation rates at 1, 3, and 5 years were 16% (95% CI: 7-25%), 3% (95% CI: 0-11%), and 2% (95% CI: 0-5%), respectively. The median survival time (Table 3) was 0.3 years (95% CI: 0.2-0.4%).
Sub-cohort 2. patients with 0.1 < v ≤ 0.4 before brachytherapy. low-medium
Seventy-three of the 305 patients were included in this sub-group (24% of the total cohort). The preservation of the organ was achieved in majority of patients (87%), and 10 of 73 patients were eventually enucleated. Figure 1 shows the percentage of patients who gained VA was higher than in a previous case. A significant number of patients gained VA after being subjected to the procedure, but VA deteriorated over time.
Actuarial Kaplan-Meier curves are described in Figure 2. Visual acuity preservation rates at 1, 3, 5, and 10 years were 46% (95% CI: 36-56%), 20% (95% CI: 10-30%), 17% (95% CI: 7-27%), and 14% (95% CI: 4-24%), respectively. The median survival time (Table 3) was 0.8 years (95% CI: 0.5-1.7%).
Sub-cohort 3. patients with 0.4 < v ≤ 0.8 before brachytherapy. medium-high
Eighty-five of the 305 patients were included in this sub-group (28% of the total cohort). For patients with baseline medium-high VA (0.4 < V ≤ 0.8), the preservation of the organ was achieved in majority of patients (95%), and 4 of 85 patients were enucleated.
Figure 2 shows that in this sub-group on average, the loss of VA was greater and the number increased with time. No patient gained VA.
Actuarial Kaplan-Meier curves are described in Figure 2. Visual acuity preservation rates at 1, 3, 5, and 10 years were 65% (95% CI: 56-74%), 53% (95% CI: 45-61%), 29% (95% CI: 15-42%), and 15% (95% CI: 3-27%), respectively. The median survival time (Table 3) was 3.1 years (95% CI: 1.7-4.5%).
Patients with v > 0.8 before brachytherapy. very high
Eighty-six of the 305 patients were included in this sub-group (28% of the total cohort). For patients with high initial VA (V > 0.8), the preservation of the organ was achieved in majority of patients (93%), and 6 of 86 patients were enucleated. Tables 1 and 2 display baseline patient demographics, tumor characteristics, and doses to the tumor apex for the study population.
Figure 1 presents the loss of VA as the most numerous events. No patient improved his VA during follow-up visits. Most patients maintained their initial VA values but with time, these values were reduced till half. As in the previous cohort, no patient gained VA.
Actuarial Kaplan-Meier curves are described in Figure 2. VA preservation rates at 1, 3, 5, 10, and 15 years were 86% (95% CI: 80-92%), 56% (95% CI: 46-66%), 48% (95% CI: 35-61%), 41% (95% CI: 26-56%), and 27% (95% CI: 10-43%), respectively. The median survival time (Table 3) was 4.4 years (95% CI: 3.2-10.4%).
Log-rank test
Figure 2 shows survival curves for the relevant variables of multivariate analysis, where the curves are separated according to their initial VA. All four groups analyzed with log-rank test reported p < 0.05, so the survival curves differed significantly. Figure 2 demonstrates the median survival time for each sub-group and survival rates for 1, 3, 5 10, 15, and 20 years.
Discussion
In this report, we present our experience with VA outcomes after plaque brachytherapy in a large series of patients from a single center. The results accurately reflect the outcomes in this center, and provide a useful internal audit. A multicenter study with a larger patient population could confirm or refute the results. Visual acuity measurement can be challenging, because there are no specific standards for the type of use, for which the test is designed [31]. This highlights difficulties in comparing studies from different institutions and various patient populations. Although the organ was preserved in the majority of patients (95%), a significant number of cases experienced a decline in VA as a result of therapy. The actuarial five-year eye preservation rate was 90%. The COMS Report 18 [32] provides enucleation results of 12.5% at 5 years. The decrease and maintenance of VA, as shown in this study, depend on the initial VA of patients. In this way, patients with a low to medium VA seem to benefit the most from brachytherapy. However, on average, VA worsens over time after treatment. Patients with good baseline VA have, on average, more time remaining vision with a VAS > 50 than those with a lower baseline VA. Some of the blind or moderately sighted patients gain VA from treatment, improving their initial examination score. Therefore, VA will almost inevitably decrease after treatment, although less rapidly if the initial acuity is higher.
In cases experiencing a decline in visual acuity following cataract treatment, whether due to radiation or other factors, surgery may offer the opportunity to recover a significant portion of lost visual acuity. However, the visual outcomes of patients in our series were comparable with results of other reported studies [13, 14, 33], and the outcome of VA test was worse on average over time.
Char et al. [34] reported that the risk of vision loss was greatest immediately after treatment and decreased over time. After 3 years, 36% of the eyes had 6/12 (> 0.5) or better VA score in a retrospective analysis of 230 patients treated with brachytherapy. The COMS 18 Report 18 [32] found that 50.1% of COMS participants still had usable vision at 36 months. The results of the current study provide significantly lower values for all sub-groups investigated.
Study limitations
Uveal melanomas are linked with two primary factors contributing to vision loss, which are somewhat intertwined with exudative retinal detachment and radiation exposure. As a result, it can be challenging to attribute vision impairment solely to brachytherapy. Furthermore, anterior uveal and large posterior melanomas commonly lead to cataracts, resulting in temporary vision loss that can impact outcomes.
Our retrospective study reports the outcomes of treatment of patients with choroidal melanoma in Spain from 1996 to 2022. However, this study has several limitations. The most important is that the initial visual acuity and even the final visual outcomes, depend on several factors. For example, low initial visual acuity may depend on a small tumor near the macula with serous detachment of the fovea, or a large tumor with extensive detachment and hemorrhage. Although both patients may have the same vision at baseline, their outcomes are likely to be different. Conversely, a large tumor in the anterior segment of the eye may not interfere with central vision more than a small tumor in the mid-periphery, but again, outcomes will be different. The present study makes no attempt to account for these variables; it considers only pre-treatment vision as an explanatory variable. Second, the outcome depends on how actively and by what method unavoidable side effects of irradiation are treated, e.g., enucleation vs. anti-VEGF with cyclophotocoagulation for neovascular glaucoma, different intravitreal injections for radiation maculopathy and radiation optic neuropathy, etc. These complications and their treatment strategies (which are likely to evolve during the study period) are not described and analyzed in the manuscript.
Another limitation is that Kaplan-Meier curves behave poorly in tails, and the reliability of estimates is intuitively poor with less than 10% of patients remaining in the cohort. The second limitation is the amount of lost data in the last tracking intervals. Despite these limitations, this review provides truly valuable information on treatment outcomes in VAS after episcleral brachytherapy.
Conclusions
Most patients experience a marked worsening of their VA regardless of their VA prior to treatment with episcleral brachytherapy. Patients with a higher baseline VA best maintain VA over time. Episcleral brachytherapy cannot prevent the loss of visual acuity, especially in advanced cases, or when the tumor caused significant damage before treatment. Blindness is virtually irreversible, even when the disease is controlled locally.