Purpose
Prostate cancer is one of the most common cancers in men worldwide, with an estimated 1,600,000 cases and 366,000 deaths annually [1]. Strategies, such as radical prostatectomy, brachytherapy, or external beam radio- therapy, are commonly utilized, but have their own substantial side effects [2, 3]. Active surveillance (AS) has also become an increasingly popular approach for low- and low-intermediate prostate cancer in order to avoid side effects. However, up to 50% of men placed on AS eventually receive treatment, either because of subsequent upgrading or patient choice [4, 5]. Strategy between whole gland therapy and AS is the focal or partial gland treatment. Imaging by multiparametric magnetic resonance imaging (mpMRI) can identify a visible target from which the ablation zone can be planed [6]. Furthermore, prostate biopsy performed by transperineal mapping has enhanced lesion location accuracy and detection of clinically significant prostate cancer (csPCa), resulting in an increased likelihood of accurate target ablation [7, 8].
Additionally, focal therapy has sparked controversy within the radiation oncology community due to several potential drawbacks. These include relatively high recurrence rates, increased toxicity during salvage therapy following relapse, lack of randomized trials demonstrating its superiority over active surveillance, uncertainty about whether toxicity is genuinely lower than whole gland therapy, and challenges in unambiguous mpMRI interpretation. Nevertheless, advancements in imaging quality and biopsy techniques by the transperineal approach hold promise for overcoming only some of these issues. Furthermore, the workflow between diagnosis and treatment is not seamless, often leading to difficulty in registering and accurately targeting areas within the gland needing treatment.
Permanent prostate brachytherapy offers ideal opportunity to target specific regions of the prostate and evaluate dose parameters to the ablation zone real-time when performing a procedure [9, 10]. Furthermore, the dose required to achieve complete ablation, leading to 98% cancer control as confirmed by biopsy, has been previously reported [11]. Notably, positive biopsies occurred in 1.6% (3 out of 182) of men who received biologically effective dose (BED) > 200 Gy (p < 0.001) [11]. It is essential to emphasize that these doses, surpassing 200 Gy, were substantially higher than typical prescriptions ranging from 145 to 160 Gy for iodine-125 (125I). Consequently, this raises concerns about potential rectal injury risks for patients [12]. Therefore, a combined strategy of partial gland ablation using high-dose implant to the posterior of the gland, may also benefit from post-implant insertion of rectal spacer [13].
The purpose of this investigation using phantoms was to create a strategy with one software program to perform transperineal prostate biopsies using mpMRI with region of interest (ROI), define the margin of ablation by negative biopsies surrounding ROI and contralateral prostate, fuse the positive biopsy sites to a treatment planning module, and implantation of the region containing these sites combined with the insertion of a rectal spacer. Furthermore, documentation of implant quality, including rectal dosimetry, was accomplished using post-implant computerized tomographic (CT) dosimetry.
Material and methods
An in vitro investigation using 4 prostate phantoms (Viomerse, Inc., Pittsford, NY, USA) containing ROI of a simulated prostate cancer lesion and its DICOM MRI file were utilized. A unified approach to prostate cancer diagnosis and treatment was created by incorporating three modules from VariSeed (v. 9.0.2, Varian Medical Systems, Siemens Healthineers Company, Inc., Palo Alto, CA, USA): image fusion, transperineal biopsy (VariPath), and treatment planning; two real-time implantations with Mick and linked seeds were applied. Loose dummy seeds were pre-loaded into Mick cartridges and linked stranded dummy seeds were used for the implant and modeled as 125I sources (Brachy-Source STM 1251, Bard, Covington, GA). Hyaluronic acid (HA) (Barrigel®, Non-Animal Stabilized Hyaluronic Acid, NASHA®, Palette Life Sciences, Stockholm, Sweden) was utilized for rectal spacing. The overview of research strategy is shown in Figure 1.
All four phantoms were similar, had a clinically significant ROI located in the left posterior lobe and a benign ROI in the right upper zone, and were fixed to the procedure table in the operating room. Bi-planar trans-rectal ultrasound probe (Pro Focus 2202, BK Medical, transducer type 8848; Denmark) covered with a gel-filled condom was inserted into EXQ2 stepper (CIVCO Medical Solutions, IA), and images were acquired in axial plane from the base to apex at 5 mm intervals. DICOM images of the phantom MRI were uploaded to the image fusion program, and the prostate, rectum, and ROI were contoured (Figure 2). Transperineal biopsy procedure was then simulated using VariPath program. A comprehensive biopsy plan was performed by first dividing the prostate into quadrants with the urethra as the central axis, and then taking 32 samples between 5 and 10 mm apart using a brachytherapy grid. The biopsy procedure, performed prior to the implant procedure, consisted of 3 cores from the interior of ROI and around its periphery, 5 mm spaced samples from the contralateral lobe, and 10 mm spaced cores from the anterior of the gland. Spacing of these samples was based on a prior report [12]. Simulated positive biopsy sites in and around ROI (lateralized plan), and those containing positive results from the contralateral posterior lobe (bilateral posterior approach) were settled in the VariPath program (Figure 3). Prostate phantoms were re-scanned, and a post-biopsy VariPath file was fused with rigid registration to these new ultrasound images using VariSeed. Gross tumor volume (GTV) was defined as the ROI from MRI, and included any simulated positive biopsy sites outside of it. Clinical tumor volume (CTV) included GTV, and was defined by first negative biopsies at the periphery of ROI. Based on a study by Stone et al., the planning prescription dose of 210 Gy was applied to cover CTV [11]. Both the GTV and CTV were planned to receive a minimum of 210 Gy (Figure 4). Two different focal strategies were planned with the CTV extending into the anterior zone (a lateralized/partial hemi-ablation approach) and into the contralateral posterior zone (a bilateral posterior approach). The urethra and anterior rectal wall were delineated as organs at risk.
Fig. 2
Segmented MRI of prostate (red), urethra (green), rectum (blue), and region of interest (yellow) as gross tumor volume (GTV). Contouring was performed after fusion of prostate phantom MRI to the VariSeedTM fusion program
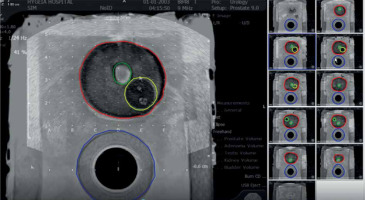
Fig. 3
Simulation of transperineal biopsy using the VariPath program. Biopsies were taken using a brachytherapy grid. Blue sites represent negative biopsies, while the red ones are positive with clinically significant prostate cancer (csPCa). The location of cancer (red) on each specimen was determined by a start point from the base end of biopsy
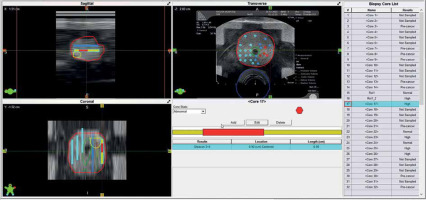
Fig. 4
Pre-planning the partial gland ablation using 9 implant needles and 27 seeds with an activity of 600 U (0.472 mCi, 125I STM 1251). Region of interest (ROI; yellow) is gross tumor volume (GTV), and orange represents clinical target volume (CTV). CTV was pathologically defined by negative biopsy sites surrounding GTV. Planned dose to CTV was 210 Gy, which entirely covered (purple isodose line) it. D90 of CTV was 254.4 Gy
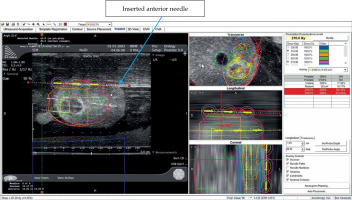
Four procedures were performed: two using Mick applicator (TP-200, Mick Radio-Nuclear Instruments, 521 Homestead Ave., Mount Vernon, NY 10550, USA) and two using strands (ReadyLinkTM Delivery System, BD, Bard, Covington, GA, USA). The prostate phantom was re-scanned, and the pre-plan was fused to the newly acquired images. After aligning the prostate, urethra, and rectum, individual needles were placed according to the pre-plan. Each needle representation was then loaded with the corresponding number of seeds according to the pre-plan. Two methods were used to align the seeds in sagittal imaging with the VariSeed program. With the Mick applicator approach, the first seed was placed just above the base end of the CTV, the second and third (if necessary) were equally spaced within CTV, and the last one at the apical end of the CTV. For the implants using strands, the corresponding seeds according to the pre-plan were similarly placed with the intent of keeping as many of the seeds within the CTV, with the base and apical ones residing slightly outside of the CTV. Each plan was then optimized by either adding or deleting seeds, or moving the needle positions with the goal of having the 210 Gy isodose cloud completely covering the CTV. In addition, dose constraints for the urethra and rectum were maintained at D30% < 150% (240 Gy in conformal prescription of 160 Gy) and RV100 < 1.0 cc % (160 Gy in conformal prescription of 160 Gy), respectively.
Following plan optimization, each needle was placed into their corresponding positions as depicted on real-time computerized plan using axial ultrasound imaging, and setting both computer and ultrasound planes at the midpoint of the prostate. If the inserted needle did not exactly match the needle representation, the representation was then re-positioned, so they were both overlying each other (Figure 5). Imaging was then switched to sagittal, and needle representations were then matched to each needle position in depth. Adjustments to seed positions were made to ensure that the prescription dose fully covered the CTV and dose constraints to the urethra and rectum were not exceeded (Figure 6). Seeds were then inserted using the Mick applicator or strands (two separate procedures). Additional matching of seed representations was done once the deposited seeds were identified on ultrasound. There were two Mick and strand procedures completed for the left partial hemi-ablation and the ablation of both posterior zones, respectively.
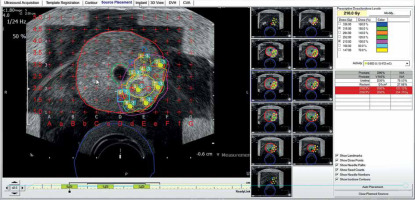
Fig. 5
Nine implant needles placed in and surrounding region of interest (ROI; blue). Red circles are positive biopsy sites that were fused to intra-operative ultrasound using VariSeed
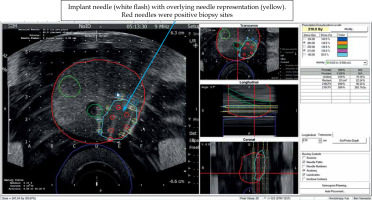
Fig. 6
Prior to implantation of sources, needle representation, as displayed on the VariSeed program was matched to inserted needle (arrow). Positions of seed representation could then be adjusted to optimize the isodose coverage of gross tumor volume (GTV) and clinical target volume (CTV). Dose values, as displayed on the right side of the screen, also informed the clinician about total coverage to CTV, urethra, and rectum
The four phantoms were then scanned with CT imaging using the following parameters: slice thickness, 3 mm; kVp, 120; reconstruction diameter, 500 mm; resolution (0.976/3.0) mm; and helix scanning. The phantoms were returned to the procedure and placed in position using an ultrasound to align the prostate and urethra with live images from the VariSeed laptop. The isodose overlays extending into the rectum were assessed, and Barrigel® was injected in a sculpted fashion to elevate the prostate away from the rectum only where rectal dose was judged to be high. After the 4 phantoms were similarly treated, they were returned to the scanner for another set of images. These procedures were conducted at the Brachytherapy Center (VS), Hygeia Hospital (Athens, Greece), using local brachytherapy setup and equipment. Several of the authors participated in the procedures (BGLV, VS, LCP, NNS).
Eight CT scan files were uploaded into the post-implant dosimetry program and evaluated at the Brachytherapy Center (Hygeia Hospital Athens, Greece), Maastro Clinic Department of Radiation Oncology (Maastricht, The Netherlands), and Mount Sinai Medical Center Department of Radiation Oncology (Icahn School of Medicine, New York, NY, USA). Post-implant dosimetry was performed on the 4 implants with 2 situations (with and without rectal spacer) at these 3 institutions, without the benefit of availability of the pre-plan or intra-operative studies.
Results
Intra-operative values
The mean prostate volume, as determined by the intra-operative VariSeed software, was 36.8 cm3 (range, 33.3-41.8 cm3). The volumes of the GTV in different phantoms and different approaches (one vs. two posterior lobes) varied from 0.6 to 1.2 cm3 (mean, 0.8 cm3), while the CTV ranged from 6.2 to 14.9 cm3 (mean, 11.3 cm3). The total number of needles necessary to implant the GTV volume varied between 9 and 13 (mean, 11). The total number of seeds (implantation was performed with dummy seeds, with assumed activity from 0.472 to 0.512 mCi) ranged from 27 to 43 (mean, 36). The mean intra-operative V100% of the GTV (volume of the GTV receiving 100% of the prescription dose) was 99.5% (range, 98.3-100%). The D90 for the entire prostate gland was intra-operatively 66 Gy (range, 32.8-104 Gy). The intra-operative V100% of the CTV (volume of the CTV receiving 100% of the prescription dose) was 91.3% (range, 86.5-95.3%). The ratios of CTV/prostate and GTV/prostate volumes varied between 19% and 42%, and between 1% and 4%, with a mean of 31% and 2%, respectively. A mean of 8.6 cc of Barrigel® was inserted (range, 7.4-10.6 cc). The mean intra-operative D90 for the GTV, CTV, prostate, urethral D30, rectal V100, and D2cc were 265 Gy, 213 Gy, 66 Gy, 199 Gy, 0 cc, and 68 Gy, respectively (Table 1).
Table 1
Intra-operative dosimetry mean values, with ranges for lateralized/partial (hemi-ablation approach) and contralateral posterior zone (bilateral posterior approach)
Post-implant dosimetry
The mean post-operative GTV, CTV, and PV from the 3 institutions were 0.8 cm3 (range, 0.6-1.2 cm3), 5.2 cm3 (range, 2.2-9.9 cm3), and 37 cm3 (range, 28.6-42.6 cm3), while the mean post-operative focal D90 of the GTV and CTV were 235 Gy (range, 208-251 Gy) and 213 Gy (range, 210-216 Gy), respectively (Table 2).
Table 2
Comparison of post-operative implantations without and with rectal spacer dosimetry mean values, with ranges
Comparison of the intra-operative values with the post-implant means from the institutions are shown in Table 3. In general, the intra-operative values were very similar to the post-operative values with one exception. In one of the Mick applicator procedures, post-operative dosimetry revealed that one needle was not implanted as planned reducing the planned dose for D90 of the GTV and CTV from 234 Gy and 223 Gy to 195 Gy and 183 Gy, respectively.
Table 3
Comparison of intra-operative planning values with post-implant means without spacers from different institutions
Rectal spacer insertion
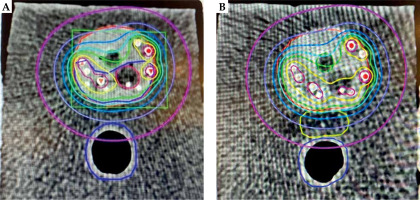
On average, 13 mm separation was achieved between the prostate and the rectum (range, 10-14 mm), with a mean of 8.6 cc spacer implanted (range, 7.4-10.55 cc). The mean post-operative doses in Gy to 0.1 cc of the rectum (D0.1cc), to 1 cc of the rectum (D1cc), and to 2 cc of the rectum (D2cc) without spacer vs. with spacer were 63 vs. 48 Gy (24% reduction), 47 vs. 36 Gy (20% reduction), and 40 vs. 22.8 Gy (43% reduction), respectively (Figure 7).
Fig. 7
Color-wash isodose distribution in axial CT plane of a phantom prostate (red) with seeds implantation without spacer implantation (A) and with spacer (yellow) implantation (B), with gross tumor volume (GTV; hypo-dense structure – contouring dark red) and clinical target volume (CTV; dark blue). The prescribed dose to CTV was 210 Gy (green isodose). Isodose lines colors are yellow, light-blue, marine-blue, purple, and pink, with the corresponding 280 Gy (140%), 160 Gy (80%), 120 Gy (60%) 80 Gy (40%), and 40 Gy (20%), respectively. The rectum with spacer is solely covered by a small isodoses of 40 Gy
Discussion
Using prostate phantoms, the investigators created a novel approach to focal and partial gland ablation utilizing one software program for mpMRI fusion-guided transperineal biopsy, treatment planning, and intra-operative dose adjustment. Fusion biopsy steps are commonly separated from the treatment plan, often relying on cognitive fusion of biopsy results and estimation of ablation field from these. Coordination between these two, as demonstrated herein, has the potential to increase planning accuracy. In addition, we modeled the margin for ablation by relying on negative biopsies and inputting these into the planning software. To our knowledge this is the first time ‘pathologic’ data has been demonstrated to be beneficial in guiding margin determination.
Two issues that are a major concern to clinicians considering focal therapy are in-field and out-of-field recurrences (IFR and OFR). They are exemplified by less favorable results reported from centers utilizing cryoablation, laser, and high-intensity focused ultrasound (HIFU) [14-18]. Tourinho-Barbosa et al. analyzed 309 patients, including 190 treated with high-intensity focused ultrasound and 119 treated using cryotherapy [14]. In 285 patients (92%) who underwent post-FT biopsy, 122 (42.8%) were positive. IFR were found in 101, and OFR in 55 cases. Mortezavi et al. performed an extensive biopsy protocol (44 samples) 6 months following HIFU in 75 men [15]. Clinically significant PCa was detected in 28 of 68 patients (41.2%). In 14 of the 68 cases (20.6%), csPCa was evident in the treated area.
One of the goals of the present study was to utilize the unified approach to minimize both IFR and OFR. OFR should be decreased by accurately identifying the tumor margin by extending the ablation zone to negative biopsies collected, which surround the ROI, and by the mapping procedure in regions contralateral to the ROI. Because the risk of clinically significant disease associated with PI-RADS 4-5 lesions is firmly established, biopsy of that quadrant can be limited to 2 or 3 cores from the ROI and to biopsy of the lesion perimeter [19]. A quadrant approach can further help reduce the number of biopsies required. After biopsy of the ROI, 5 mm samples taken along the horizontal axis (passing through the urethra) can help define the margin, and eliminate the need for additional biopsies within this posterior quadrant. If these are negative, the ablation zone can spare the anterior zone above (assuming the 10 mm spaced biopsies in the corresponding anterior zone are also negative). However, on the contralateral posterior quadrant, a more extensive biopsy plan should be considered, because as Stone et al. has shown, biopsies taken at 5 mm intervals can identify cancers as small as 0.1 cc [10].
In-field recurrence results from an inadequate ablative energy of HIFU within the CTV. While radiation therapy is thought to be equivalent to radical prostatectomy when whole gland therapy is utilized, its use for focal ablation has received far less investigation. Laing and colleagues compared intra-operative and post-implant dosimetry of hemi-gland implants with a conventional prescription dose of 145 Gy. The mean post-operative D90 for the target hemi-gland was 153.8 Gy compared with 47.5 Gy for the contralateral hemi-gland [20]. A clinical pilot study by Langley and co-authors suggested that treatment-related toxicity and biochemical outcomes after hemi-gland implants were comparable with whole glands: sexual potency was preserved in 73% and 67% of hemi- and whole gland groups, respectively (p = 0.84). Treatment relapses in both the groups were observed as 3% [21]. Cosset et al. reported on twenty-one patients with focal therapy and prescription dose of 145 Gy [22]. The mean focal D90 was 183.2 Gy (range, 176.4-188.1 Gy), with a D90 for the entire prostate of 33.6 Gy (range, 18.7-57.9 Gy). Only minimal acute toxicity was observed, but no data of long-term toxicity was available [22]. Studies of Langley et al. [21] and Cosset et al. [22] used traditional prescription doses of 145 Gy. However, radiation doses equal to or above a BED of 200 Gy have demonstrated improved outcomes, Stone and others have published long-term data on biopsy results, which demonstrated that a biological equivalent dose (BED) of 220 Gy (using an α/β of 2) can result in a negative biopsy rate of 98.4% [23, 24]. This equates to a dose of 210 Gy when 125I is used for brachytherapy. We relied on these dosing data to model the current study that showed it was possible to achieve high intra-prostatic doses when directed to the CTV, while keeping the total gland doses low (Figure 7).
To achieve doses exceeding 210 Gy in focal brachytherapy, dose escalation to the tumor could lead to increased rates of toxicity. Given that most prostate cancers are located in the posterior of the gland and could involve the capsule, achieving an ablative radiation dose in this region could put the rectum at additional risk [25]. To mitigate the risk to the rectum, an implanted rectum spacer, acting as a bio-degradable tissue filler, can be inserted between the prostate and the rectum. This increases the distance between the anterior rectal wall and the prostate, effectively moving the entire anorectal structure out of high-dose region [26]. However, in a focal strategy, it may not be necessary to displace the entire rectum from the high-dose region, as localized sculpting of the rectum spacer to the region of interest could be sufficient. We showed the feasibility of this approach in phantoms by comparing post-implant rectal dosimetry before spacer insertion with post-insertion dosimetry. While several studies have demonstrated rectal separation in patients about to undergo external beam irradiation, this is the first investigation to specially show that spacer insertion can be done post-implant with a sculpted technique, where pre- and post-spacer insertion dosimetry are compared. These data suggest that if intra-operative dosimetry is used during brachytherapy, the need to increase prostate to rectum distance can be made at the end of procedure. Unnecessary spacer insertion and the amount of material to be inserted when sculpting could limit complications associated with current practices [27].
One of the consequences of achieving doses to the CTV of 210 Gy or higher are the concomitant high doses to small volumes of the urethra. The mean D30 to the urethra in the phantoms ranged from 164 Gy (for the lateralized approach) to 254 Gy (for the bilateral posterior procedure). These doses are relatively high when compared with standard parameters for whole gland brachytherapy. It is recommended to adhere to dose constraints provided by the American Brachytherapy Society (ABS) and the Groupe Européen de Curietherapie - European Society for Radiotherapy and Oncology (GEC-ESTRO) [28, 29]. In future trials, the following dose constraints for the urethra should be considered: the prostatic urethra D10% < 150% (217 to 240 Gy) and D30% < 130% (189 to 208 Gy), according to the ABS and GEC-ESTRO. While the mean values from this study fall below these thresholds, some phantoms may exceed these values, which warrants careful consideration when clinical trials are considered.
This study has some limitations. It was an in vitro study, and will need to be validated in clinical trials. In one plan, a needle was not inserted and had an incremental impact on the dose: the D90 of the GTV and CTV intra-operative were planned as 234 Gy and 223 Gy; however, post-operative revealed doses of 195 Gy and 183 Gy, respectively. While this is still in the area of traditional prescription doses of 145 Gy, it shows the importance of adhering strictly to planning, what is even more important in this focal approach in comparison with whole gland strategies. Additionally, the investigators involved in this study, including radiation oncologists, urologists, and physicists, possess extensive experience in brachytherapy and transperineal biopsy. When treating a focal volume, it becomes even more critical for the clinician to accurately target the intended area, as compared with a whole organ strategy, where potential mismatches are more forgivable. Therefore, a learning curve for implementing such an approach is essential to ensure precise and effective treatment. In addition, integration of the 3 modules used in this investigation, including image fusion (for mpMRI), biopsy mapping, and fusion of the biopsy results to the planning software, are not intuitive. The significant importance of the benefit of rectal dose reductions in phantoms is also undervalued. In real clinical practice, the distance between the prostate and the rectum in patients is frequently a few millimeters, which contrasts with phantoms used for educational purposes, where the distance can exceed 5 millimeters or more. More rectal dose reductions should be expected in vivo. Additionally, 5 mm interval planes are relatively spaced for this high precision technique, which could be solved by 1 mm slices and/or a tracked-stepper technique. Moreover, by implantation of needles, oedema can affect the shape of the prostate. Further research must confirm if this is valuable, and if an adaptation strategy for CTV is necessary.
Lastly, individuals contemplating their own investigations will need to be trained in the entire workflow prior to clinical work. Practicing with prostate phantoms before treating patients may provide a benefit in comparison with the traditionally master-apprentice model, with typical shortcomings relating to patient safety, as the training is performed with real patients.
Conclusions
The feasibility of a unified approach to the diagnosis and focal treatment of prostate cancer utilizing brachytherapy in prostate phantoms is demonstrated in the current study. It is possible to deliver ablative radiation doses to the CTV and protect the rectum with a spacer insertion. Data from this investigation may allow for the design of clinical studies incorporating these principles.