Introduction
Renal cell carcinoma (RCC) is the most commonly diagnosed kidney cancer and accounts for 90% of all kidney malignancies [1]. It represents around 2% of all cancer cases and cancer-related deaths worldwide [2]. Clear cell carcinoma represents around 70% of all histological subtypes of RCC and usually develops on a background of different sporadic mutations including VHL mutation [1]. VHL gene expression plays a crucial role in the physiological response to hypoxia at the cellular level; hence abnormalities of its function can lead to cancer development [1]. Under those circumstances, HIF proteins (HIF-1α, HIF-2α, and HIF-3α) accumulate and via numerous molecular pathways lead to the promotion of angiogenesis and cell proliferation, important elements of carcinogenesis [3, 4]. Moreover, HIF-1α is known to induce expression of vascular endothelial growth factor (VEGF) [1] and may cause upregulation of MET and AXL [5–8]. Increased expression of MET or AXL is related to poor prognosis [9, 10] and decreased response to VEGF receptor inhibitors in the preclinical model of RCC [10, 11]. Therefore, it is considered essential in RCC development [3].
Understanding these pathways has allowed the implementation of tyrosine-kinase inhibitors (TKI) in the systemic treatment of RCC [1]. Tyrosine-kinase inhibitors such as pazopanib, cabozantinib, sunitinib, and axitinib cause an antiangiogenic effect by targeting the VEGF receptor [12] and in sequence interfering with the growth of the tumor [1]. Unfortunately, with time the majority of patients will inevitably acquire resistance to these treatments [3, 12].
Cabozantinib is an oral inhibitor of MET, AXL, and VEGF receptors that also inhibits other receptors and kinases such as RET, KIT, and ROS1. It affects the physiology of the tumor and leads to apoptosis of tumor cells, disruption of tumor vascularization, and increased hypoxia within the tumor. Vascular endothelial growth factor inhibition is responsible for some of the immunomodulatory effects, as VEGF has immunosuppressive properties, such as increasing the number of myeloid-derived suppressor cells (MDSC) and regulatory T-cells [13–15]. Cabozantinib affects not only the tumor microenvironment but also directly acts on the tumor cells, making them more susceptible to immune-mediated killing [16]. These features are the reason for the occurrence of a synergistic effect with immune-oncology (IO), which has already been described in various studies [17].
Sequencing therapy, in which cabozantinib plays a crucial role, is especially important in patients with metastatic renal cell carcinoma (mRCC). Nowadays it is used as a second or further line of treatment after the failure of the preceding line. It is also effective after progression in patients pretreated with other VEGF inhibitors (VEGFi) as it breaks resistance to previous therapy [18]. Recent ESMO [19] and ASCO [20] guidelines recommend using IO combinations (IOIO) or IO-VEGFi combinations (IOVE) [21–23] as first-line treatment in advanced RCC. Despite the lack of good- quality evidence, some authors suggest that using cabo-zantinib after the failure of IO, IOIO, or IOVE is possible [24–27]. In the CABOSUN study cabozantinib demonstrated effectiveness as a first-line treatment in intermediate- and poor-risk patients according to International Metastatic RCC Database Consortium (IMDC) criteria [28, 29]. It is also the preferred first-line agent for advanced papillary RCC without additional molecular testing [19].
The aim of our study was to assess the effectiveness of cabozantinib in the real-world setting in pre-treated patients. We were especially interested in whether a subgroup of patients previously treated with multiple lines, including TKIs, would benefit from cabozantinib. Moreover, we wanted to check what factors can potentially influence the efficacy of the treatment.
Material and methods
Patients
This retrospective analysis included seventy-one patients with biopsy-proven mRCC undergoing cabozantinib treatment as a second or further line at the Department of Genitourinary Oncology of the Maria Skłodowska Curie National Research Institute of Oncology in Warsaw. The database contained the data of patients with mRCC treated at the department between 30th January 2017 and 23rd June 2021. This study was performed in line with the principles of the Declaration of Helsinki. Permission to conduct this study was granted by the Maria Sklodowska-Curie National Research Institute of Oncology Bioethics Committee (permission number 38/2018).
Data collection
The database contained detailed information on age, gender, clinicopathological factors, laboratory results, comorbidities, adverse events, sites of metastases, Eastern Cooperative Oncology Group performance score, IMDC and Memorial Sloan-Kettering Cancer Center (MSKCC) risk scores [28] and outcome data associated with individual patients. Clinical data were extracted from medical records and mortality data were obtained from the Polish national database. Detailed characteristics of the studied group at the time of the start of the study are shown in Table 1.
Table 1
Characteristics of studied group at start of study (at initiation of cabozantinib treatment)
The study included patients aged between 42 and 80 years with biopsy-proven metastatic renal cancer. Other than clear cell morphology did not exclude patients from the study, who were treated with cabozantinib as second or further-line treatment. The initial dosing of cabozantinib was 60 mg per day for all patients. Dose modifications were based on the Summary of Product Characteristics [30]. Each patient should have had the complete blood counts evaluated before starting the course of treatment with cabozantinib. Hematological parameters were measured using the Sysmex XN-1000. Laboratory tests were carried out by the Diagnostic Department of the National Research Institute of Oncology. The patients were classified into three groups – favorable, intermediate, and poor risk – both for MSKCC and IMDC, according to the score they got, but none of this group was excluded from this study. Adverse events were assessed in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [31]. Tables 2–4 show the previous lines of treatment, and Figure 1 illustrates the applied treatment regimen.
Table 2
Line of cabozantinib use
| Cabozantinib as 2nd line treatment, n (%) | 30 (42) |
|---|---|
| Cabozantinib as 3rd line treatment, n (%) | 36 (51) |
| Cabozantinib as 4th line treatment, n (%) | 4 (6) |
| Cabozantinib as 5th line treatment, n (%) | 1 (1) |
Table 3
Detailed previous treatment
Inclusion criteria
Biopsy-proven metastatic clear cell renal cell carcinoma.
Biopsy-proven metastatic non-clear cell RCC if other treatments failed – emergency access to drug technologies.
Cabozantinib treatment in the second or further line with the initial dose of cabozantinib 60 mg per day.
Consent to treatment and participation in the study.
General condition and laboratory parameters allowing for systemic treatment.
This study included patients eligible for systemic treatment with biopsy-proven metastatic clear cell renal cell carcinoma. Patients with non-clear cell RCC were also included if they received cabozantinib through emergency access to drug technologies due to failure of other treatment options. Moreover, cabozantinib had to be administered as a second or further-line treatment with an initial dose of 60 mg per day. Consent to treatment and participation in the study was also required.
Endpoints
The primary endpoints of the study were progression- free survival (PFS) and overall survival (OS) for patients treated with second or further-line cabozantinib. Secondary endpoints assessed the best overall imaging response (BOR) to cabozantinib, as per RECIST v.1.1 [32]. Patients evaluable for response were defined as those who had baseline imaging and at least one set of imaging studies after initiation of cabozantinib treatment.
Statistical analysis
Categorical variables were summarized with the number and percentage of the respective group. Quantitative variables were summarized with mean and standard deviation (normally distributed) or median, first and third quartile (Q1; Q3; non-normally distributed) as specified in Results. The subgroups were compared with the Pearson χ2 test, Fisher exact test (2 × 2 tables, expected frequency < 5), Student t-test, or Mann-Whitney U test. Progression-free survival times were calculated from the date of initiation of cabozantinib (i.e. the start of the study) until the date of diagnosis of progressive disease (PD), death, or were censored on the date of loss to follow-up or the end of the study (5th February 2022). Overall survival times were calculated from the date of initiation of cabozantinib (i.e. the start of the study) until the date of death, or censored on the date of the end of the study (5th February 2022). Survival times were estimated with the Kaplan-Meier method and compared between the groups using log-rank tests. Cox proportional hazard regression was used to verify the associations between the treatment modalities preceding initiation of cabozantinib and survival (PFS and OS); the analyses including all studied patients were adjusted for the line of treatment with cabozantinib (2nd vs. further-line). All the statistical tests were two-tailed and the results were interpreted as significant at p < 0.05. Statistica software (version 13; Tibco, Tulsa, OK, USA) was used for computations.
Results
The study included seventy-one patients aged between 42 and 80 years, between 4 months to 19 years (237 months) from RCC diagnosis (median 52 months), who have been treated between 30th January 2017 and 23rd June 2021. Thirty of them (42%) were receiving cabozantinib as a second-line treatment (2L), thirty-six (50%) as a third-line treatment, and only five (7%) as a fourth- or fifth-line treatment. The details of the treatment are shown in Figure 1 and Tables 2–4.
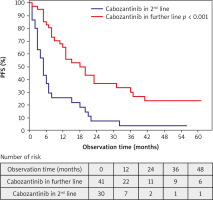
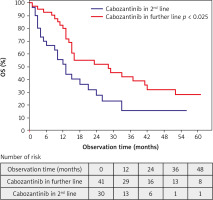
Observation time (from the start of the initiation of treatment with cabozantinib until death or the end of the study at 5th February 2022) was between 1 and 61 months; median (Q1; Q3): 15 months (95% CI: 9–31). Progression was observed in 55 (77%) patients. Progression in the second line and further line treatment was observed in 28 (93%) and 27 (66%) patients, respectively (p = 0.006). Forty-seven (66%) died before the end of the study. Sixteen (23%) patients who did not progress continued cabozantinib at the end of the study. Median PFS was 11 months (95% CI: 5–23) and median OS was 16 months (95% CI: 10–42). Median PFS in 2L and further line treatment was 5 months (95% CI: 3–9) and 18 months (95% CI: 8–39), respectively (p < 0.001). Median OS in 2L and further line treatment was 12 months (95% CI: 4–25) and 26 months (95% CI: 13–NR), respectively (p = 0.025). Partial response as the BOR was observed in 1 (3%) patient in 2L and 13 (32%) patients in further line treatment (p = 0.012) (Figs. 2, 3, Tables 5–7).
Table 5
Results of treatment with cabozantinib
Table 6
Earlier treatment (i.e. before cabozantinib) as a predictor of progression-free survival and overall survival. The analysis was performed in the whole studied group and the results were adjusted for the line of cabozantinib treatment: 2nd vs. further line
Table 7
Earlier treatment (i.e. before cabozantinib) as a predictor of progression-free survival and overall survival in 41 patients receiving cabozantinib in 3rd or further line
Adverse events and dose reduction were also assessed in multivariate analysis but there were no statistically significant differences in second and further line treatments. Adverse events occurred in almost every patient, with 65 (92%) of them experiencing at least one. The most common AE was hypothyroidism (n = 35; 49%), followed by hand-foot syndrome (n = 33; 46%). Dose reduction was necessary in 35 (49%) cases due to toxicity. Cabozantinib was generally well tolerated, with no new safety concerns or treatment-related fatalities identified. Detailed information is available in Table 8. There were no statistically significant differences in the number of metastases between the second and subsequent lines of treatment, except for pancreatic metastases – 0/30 (0%) in the second line and 6/41 (15%) in the subsequent lines (p = 0.036).
Discussion
This study describes the efficacy and treatment outcomes of cabozantinib in a real-world mRCC patient population. The obtained results indicate that cabozantinib, regardless of the line of systemic therapy in which it was used, had antitumor activity and this efficacy increased with the number of previous treatment lines. It is important to consider that these findings could be attributed to a less aggressive phenotype of disease in patients who have undergone the third and subsequent lines of treatment and survived. Notably, a higher proportion of patients with bone metastases was observed in the group receiving cabozantinib as subsequent lines of treatment in comparison to the group receiving it as a second line (42% vs. 23%). This could contribute to the greater activity of cabozantinib, as it has been proven as an effective drug in such a subgroup of patients [33]. Moreover, pancreatic metastases, which are associated with a more indolent course of the disease, were also more common in patients receiving cabozantinib as subsequent lines of treatment [34]. However, these factors are also influenced by the fact that metastases inevitably occur during the course of the disease, and the longer the survival of treated patients, the more metastases will develop.
The objective response rate (ORR) in 2L and further lines (3% and 32%, respectively) in our study appeared to be more scattered than in other studies [18, 35–38]. However, considering the ORRs of all the patients (20%), it is consistent with the ORR (17%) reported in the METEOR study by an independent radiology committee [18]. The exceptionally low ORR in 2L might have been related to the small sample size or was a matter of chance. It should be noted that the percentage of PD remained at a similar level in both groups (15% in 2L and 17% in further lines).
There are many real-world experience studies in the literature with cabozantinib that confirm its effectiveness, and the results of this study are consistent with them. In the Polish managed access program (MAP), in the group of 115 patients treated with cabozantinib in 2L or further lines the ORR was 19%, and the median PFS was 12.5 months (95% CI: 9.2–14.2 months) [37]. Furthermore, the expanded access program for cabozantinib in the UK showed an ORR of 26%, median PFS of 7.7 months (95% CI: 5.3–10.1), and mOS of 9.1 months (95% CI: 6.6–11.6) [36]. Procopio et al. in the Italian MAP study observed a shorter PFS of 8.0 months (95% CI: 0.5–10.8 months). However, ORR was 36% [38].
There was some heterogeneity in our study population, given that 49 patients (69%) were exposed to TKI, whereas 15 patients (21%) were exposed to mTOR inhibitors, and 7 patients (10%) received immunotherapy as the treatment directly preceding cabozantinib. Throughout the study, 11 patients (15%) received immunotherapy in previous lines of treatment (Tables 2–4), but only two patients (3%) received IOIO and none received IOVE, which are considered a current first-line treatment [19, 20]. There is more and more evidence of cabozantinib efficacy after IOIO and IOVE [24–27]. The efficacy of cabozantinib after IOIO and IOVE is currently being researched [24–27]. The non-randomized BREAKPOINT trial included forty-eight patients receiving IO in 1L. The median PFS was 9.3 months and the ORR was 43% [39]. The CANTATA trial, which compared the glutaminase inhibitor telaglenastat + cabozantinib vs. placebo + cabozantinib, confirmed the effectiveness of cabozantinib in the population that was treated with IPI-NIVO. Pre-specified analysis of the post-IPI-NIVO subgroup showed a median PFS of 9.2 months, with an ORR of 37%. There was no improvement in the antitumor activity of cabozantinib in the telaglenastat + cabozantinib group [40].
The immunomodulatory effect of cabozantinib may play an important role in the peri- and post-immunotherapy setting. Due to VEGF inhibition, it causes a decreased number of MDSC and regulatory T-cells [13–15]. Cabozantinib alters the tumor microenvironment and also acts directly on the tumor cells, making them more susceptible to immune-mediated killing [16]. It should be noted that the ideal sequence of therapy and optimal further lines of treatment are still being investigated [41].
Despite the small group of patients treated with drugs different than TKI in the first line, we obtained statistically significant results demonstrating higher effectiveness of cabozantinib when TKI was the first line of treatment (Table 6). These favorable results support the hypothesis that the clinical activity of cabozantinib in RCC may result from targeting the angiogenesis of the tumor (by blocking the VEGFR pathway), the mesenchymal transition (by blocking the MET pathway) and the AXL pathway. It should also be mentioned that the longer the tumor develops, the more the expression of HIF-1α decreases. This, subsequently, may reduce the importance of VEGF and lead to an increased role of MET and AXL.
Results of the CaboPoint study indicate better ORR in the TKI naïve group than the IOVE group (31.7% vs. 25%) [42]. In contrast, but consistent with our findings, the CABOSEQ study showed different results [26]. The best ORR occurred after IOVE treatment (32.5%). Objective response rates after IOIO and pazopanib/sunitinib were comparable (26.4%; 25.2%) [26]. Moreover, the longest time to treatment failure (TTF) occurred after sunitinib/pazopanib in the first line. However, statistical significance was not observed and Navani et al. concluded that there was comparable activity of 2L cabozantinib regardless of prior 1L therapy [26]. There might have been a bias due to the different characteristics of these groups. Contrary to Shah et al., we did not observe an increase in PFS in the IMDC intermediate-risk group [27].
The phenomenon of greater activity of cabozantinib in further lines of treatment is unclear and difficult to explain. However, AXL expression may increase with the clinical stage of the disease [43]. AXL is also positively correlated with PD-L1 expression in aRCC, so inhibition of AXL may potentially be responsible for reducing PD-L1 expression and stimulating the immune system [44]. However, it should be noted that we do not know whether AXL expression directly influences PD-L1 expression. This is our proposed hypothesis, but studies confirm that AXL correlates with resistance to IO and has an immunosuppressive effect, for example by promoting macrophage polarization towards an immunosuppressive pro-tumor M2-like phenotype [45–47] Notably, the prevalence of bone metastases among patients in further lines of treatment also may have benefited from the activity of cabozantinib, as mentioned above.
Limitations of this study include the small size of the subgroups, the use of descriptive statistics, the heterogeneity of patients, and the retrospective nature of the study. Moreover, the single-centered nature of the study further limits the conclusions that can be derived from it. More research should be conducted to assess the effectiveness of cabozantinib after disease progression, especially after treatment with the current first-line treatment – IOIO, IOVE, and in a multi-center setting. The results provide a benchmark for future real-world studies in mRCC.
Conclusions
Cabozantinib shows antitumor efficacy in the second and subsequent lines of treatment. The further the line of treatment, the better the effectiveness of cabozantinib was observed in this study. Nonetheless, these results should be interpreted carefully because it may be a bias associated with a less aggressive form of cancer in people receiving further lines of treatment and the prevalence of bone metastases among patients in further lines of treatment. Cabozantinib was more effective in patients treated previously with TKIs as a 1L treatment compared to other types of 1L treatment. These findings still require further research, as there are studies showing opposite findings with no such correlation. There were no other significant correlations between the choice of treatment in the previous lines and the efficacy of cabozantinib.