Introduction
In 1992 Gibbs et al. described successful stenting of the patent ductus arteriosus (PDA) in two neonates with complex pulmonary atresia [1]. Since then the procedure has increasingly been performed in preference to the modified Blalock-Taussig shunt (mBTS) with potential advantages of avoiding cardiac surgery in the neonatal period and stimulating better and more symmetrical pulmonary arterial growth [2–5].
Variations in the site of ductal origin, insertion, shape and tortuosity lead to an unpredictable array of ductal morphologies, with complex duct-airway interactions which are poorly understood [6]. In this report we describe two such scenarios wherein PDA stenting led to severe airway obstruction and required removal of the stent.
Case studies
Case 1 – baby A
A 2-week-old boy (weight 3.7 kg) with an antenatal diagnosis of right atrial isomerism, unbalanced atrioventricular septal defect, supracardiac total anomalous pulmonary venous drainage and pulmonary atresia was stable on the ward with prostaglandin infusion maintaining ductal patency. Computed tomography (CT) of the thorax showed a right-sided aortic arch with the duct in close proximity to the left main bronchus. The pulmonary arteries were confluent (left pulmonary artery (LPA) 5.5 mm, right pulmonary artery (RPA) 4.5 mm) and supplied by a long straight duct originating from the base of the left innominate artery, inserting towards the left pulmonary artery.
PDA stenting was performed under general anaesthesia via the right femoral artery. The PDA was crossed with a 014” BMW and 014” Thruway wire with resulting ductal spasm and desaturation leading to instability. A 4.5 × 24 mm Liberté stent (Boston Scientific, Natick, Massachusetts) was deployed at the PA end to restore pulmonary blood flow. A second 4.5 × 20 mm Liberté stent was then deployed to cover the aortic end. There was some migration of the first stent towards the PA; hence a third 4.5 × 16 mm Liberté stent was required to overlap the stents. The final angiogram showed good flow to both pulmonary arteries. Though there was some protrusion there was good flow in the innominate artery. The procedure time and fluoroscopy times were 75 and 17 min respectively.
Post-procedure saturations were 85%. He developed necrotising enterocolitis which was medically managed and he was subsequently discharged home 16 days after the procedure on antiplatelet therapy.
Due to recurrent desaturations and lower respiratory tract infections he had multiple prolonged hospital admission requiring periods of non-invasive respiratory support. At 6 months of age he remained an inpatient unable to wean from respiratory support.
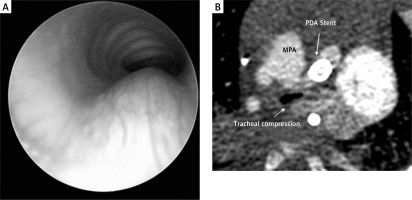
Bronchoscopy showed severe tracheomalacia with external compression of the trachea (Figure 1 A). CT scan confirmed anteroposterior (AP) compression of the trachea by the ductal stent (Figure 1 B). On retrospective review of the data it was evident that the ductal stent had formed a vascular ring due to right arch and stented left duct. This is a previously unreported iatrogenic creation of a vascular ring leading to airway compromise.
Figure 1
A – Following stent placement bronchoscopy showed external compression of the trachea. B – CT showing AP compression of the trachea by the stented ductus
The child underwent creation of a right ventricle to pulmonary artery conduit with removal of the stented duct. There was subsequent resolution of bronchial compression.
Case 2 – baby B
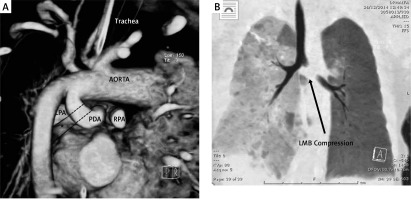
A 4-week-old boy (weight 3.8 kg) with an antenatal diagnosis of tricuspid atresia, ventricular septal defect and pulmonary atresia was referred to our unit for consideration of ductal stenting. CT showed a large tortuous PDA arising from the underside of a left-sided aortic arch and inserted into the proximal LPA with evidence of proximal LPA stenosis (proximal and distal LPA measured 3.4 mm and 6.7 mm respectively, RPA 5.6 mm). The acute midsegment turn of the PDA appeared to compress the left main bronchus (Figure 2 A).
Figure 2
A – 3D rendering of CT scan looking at the proximal part of the PDA and pulmonary arteries from behind. The PDA arises from underneath the aortic arch. It forms a gentle curve proximally but turns acutely at mid-segment, compressing the left main bronchus anteriorly. B – Post operative CT showing compression of left bronchus with good airway patency distally. The discrepancy in lung aeration was also noted on the chest X-ray
PDA stenting was performed via the femoral artery under anaesthesia. A 4 × 18 mm Azule stent (OrbusNeich Medical, The Netherlands) was inadvertently positioned slightly towards the LPA. The final angiography showed a good stent position with balanced flow to the LPA and RPA. The procedure and fluoroscopy times were 60 min and 15 min respectively. The patient was weaned and extubated after 48 h. However, he developed increasing respiratory distress requiring re-intubation with further failed attempts at extubation.
On review of the pre-procedure CT the left main bronchus appeared to be compressed by the large body of the PDA, which has an acute curvature in the middle segment. There was good airway patency distally. During the procedure, stenting and straightening the PDA led to significant narrowing of the left main bronchus evidenced by progressive hyperinflation of the left lung (Figure 2 B).
To address this problem the patient underwent creation of a 3.5 mm right-sided mBTS, removal of the stent and PDA division by a median sternotomy. He was extubated 2 days after the BT shunt surgery and transferred back to the referring hospital for ongoing care.
Discussion
Safety and feasibility of PDA stenting for augmenting pulmonary blood flow have led to its widespread application. The technique is standardised and refined to successfully deal with multiple variations of PDA morphology and minimise the risks of acute thrombosis, spasm, stent migration and perforation [2, 7]. Increased ductal tortuosity is a predictor for procedural failure. The carotid or axillary approach has proved to be an effective direct route into a vertical ductus to improve chances of successful stent deployment [7–9].
There is however very little information about the effect of ductal stenting on complex PDA-airway interactions within the superior mediastinum. A PDA arising from the contralateral side to the aortic arch or aberrant (retroesophageal) subclavian artery can potentially create an obstructive vascular ring. Careful evaluation of the PDA and its relationship with major airways and the oesophagus in the superior mediastinum is mandatory in order to predict and potentially avoid serious complications as the ductal stenting programmes expand all over the world.
Right aortic arch with mirror image branching pattern occurs due to involution of the dorsal segment of the left arch between the left subclavian artery and descending aorta. The right ductus arteriosus also undergoes involution and the remaining left ductus arteriosus usually originates from the left innominate artery or subclavian artery [10]. This configuration may form a vascular ring, with stenting potentially resulting in airway compression and significant respiratory morbidity.
The pre-procedural CT scan is useful in case selection and procedural planning. Airway proximity to the duct should be noted and although this would not necessarily preclude stenting, vigilance is required during follow-up. In a recent single-centre study, dilated and tortuous ductus arteriosus compressing the left mainstem bronchus was found in 4.6% of cases (3/64) being evaluated for ductal stenting or mBT shunt [11]. Diagnosis of airway compression was made following CT and bronchoscopy. These patients proceeded to division of the PDA and placement of a BT shunt in view of the concerns of exacerbating pre-existing airway compression. Decision making in these cases is difficult with potential shrinkage, straightening and lateralization of the PDA which may potentially improve pre-existing bronchial compression. This is an approach taken in 4 cases where airway compression improved following stenting of a large tortuous PDA with pre-existing airway interaction. No new respiratory symptoms developed in these cases [12].
Case 2 highlights the difficulty in decision making when faced with a large tortuous duct and pre-existing airway compression. Despite successful stenting of the duct airway compression persisted. In retrospect we felt the duct originated from the posterior aspect of the arch (Figure 2 A) instead of the under surface, making it closer to the left main bronchus than the usual vertical PDA. Its close proximity and large size caused dynamic compression of the left main bronchus following stenting (Figure 2 B). Persistent desaturation or respiratory distress necessitates meticulous exclusion of airway compression. Differential aeration on CXR can raise suspicion of airway compression. Earlier stent removal may need to be considered. Virtual 3D modelling may help in case selection as well as follow-up in patients in whom there are concerns about airway patency [13].
Conclusions
We describe a rare complication of ductal stenting. With the growing experience of ductal stenting and willingness to stent in children with complex heart lesions we recommend careful pre-procedure assessment of ductal-airway relationships. There should be a low threshold for CT and bronchoscopy in patients with respiratory symptoms following ductal stenting.








