Based on the United Network for Organ Sharing (UNOS) database, 3 329 patients underwent heart transplantation in the United States in 2021. The development of new surgical techniques and monitoring methods, along with new immunosuppressant drugs, has significantly increased the survival of heart transplant patients [1, 2]. Thus, the number of patients who require non-cardiac surgery after heart transplantation is undoubtedly increasing [1, 3].
Heart transplant recipients present unique perioperative challenges, given their changes in anatomy, physiology, pharmacokinetics, and pharmacodynamics [4]. Specifically, sympathetic and parasympathetic denervation of the heart leads to an altered response to many important anaesthetic medications. Neuromuscular blocking agents (NMBAs) are a class of medications that antagonise acetylcholine receptors and facilitate surgery. Neostigmine (NEO) is an acetylcholinesterase inhibitor that antagonises the effect of NMBA by preventing the breakdown of acetylcholine while sugammadex (SGX) is a modified γ-cyclodextrin that encapsulates steroidal NMBAs and antagonises their effect. After denervation, NEO-induced acetylcholine increase does not have the same effect on transplanted hearts compared with non-transplanted hearts [5]. How-ever, a range of effects from no response to asystole has been reported [6]. Likewise, anti-muscarinic drugs have been shown to have minimal to no response in this population, leaving some to consider avoiding NEO and glycopyrrolate in these patients [7].
Multiple studies have evaluated the effect of NMBAs in heart transplant patients, but there are no studies comparing NEO with SGX in the literature [3, 8–10]. Thus, we set out to compare intraoperative and postoperative outcomes in patients who received NEO or SGX during a subsequent non-cardiac operation following heart transplantation.
METHODS
The study was approved by the appropriate Institutional Ethical Review Board and the requirement of written informed consent was waived by the committee. The electronic medical records of adults (age ≥ 18 years) with a previous heart transplantation who underwent a subsequent non-cardiac operation under general anaesthesia and received either NEO or SGX were reviewed across our health care enterprise (3 hospitals across the United States). Patients who had undergone heart transplantation were identified using the Current Procedural Terminology (CPT) codes and were cross-referenced with pharmacy data on the use of either NEO or SGX from November 2015 to November 2019. Patients who signed a waiver to exclude their medical records from research studies were excluded.
Information was collected regarding patient characteristics (age at surgery, body mass index [BMI], sex, and race), heart transplant characteristics (age at heart transplant, cardiac transplantation technique, and reason for heart transplant), and surgical characteristics (time from transplant to surgery, hospital site, American Society of Anesthesiologists Physical Status (ASA-PS), pre-operative left ventricular ejection fraction [LVEF], type of surgery, estimated blood loss, vital signs before and after NMB reversal agent, presence of arrhythmias, bradycardia, hypotension, mortality, and unplanned intensive care unit [ICU] admission). Abstracted data were uploaded into and analysed using a password-protected electronic data management system (REDCap) [9].
The primary outcome of the study was to investigate the incidence of haemodynamic changes secondary to the administration of the NMBA anta-gonist including significant bradycardia and/or hypotension. Bradycardia was defined as heart rate < 60 bpm and hypotension was defined as mean blood pressure (MAP) < 65 mmHg. Secondary outcomes included the need for intraoperative inotropic agents, presence of arrhythmias, cardiac arrest, hospital length of stay (hLOS), unplanned ICU admission, and death within 30 days after surgery.
Statistical analysis
Continuous variables were summarised using the sample median and range. Categorical variables were summarised with number and percentage of patients. Comparisons of patient baseline and surgical characteristics were made using the Wilcoxon rank sum test (continuous and ordinal variables) or Fisher’s exact test (categorical variables).
Outcome variables were compared between the NEO and SGX groups using unadjusted and multivariable regression models. Continuous outcome variables were compared using linear regression models. hLOS was compared using a negative binomial regression model. The binary outcome of intra-operative hypotension was compared between the groups using logistic regression models. For outcomes variables where multivariable analysis was possible, multivariable regression models were adjusted for any baseline or surgical variable that differed between NEO and SGX groups with a P-value < 0.2. A P-value < 0.2 instead of 0.05 was used to allow the adjustment for minor differences in characteristics between the NEO and SGX groups.
For outcomes with rare events (e.g., cardiac arrest), descriptive analysis was performed. P-values less than 0.05 were considered as statistically significant, and all statistical tests were two-sided. Statistical analysis was performed using R Statistical Software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
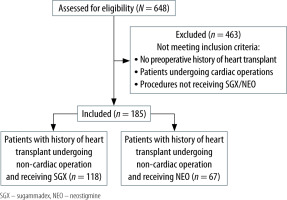
One hundred and eighty-five heart transplant recipient patients underwent non-cardiac operations at our health care system between November 2015 and November 2019. Among 185 patients, 67 patients received NEO and 118 received SGX intra- operatively at site A (n = 54), site B (n = 54), and site C (n = 77) (Figure 1).
The median (min-max) age in our study was 64 (18–80) years for NEO and 63 (23–79) for the SGX group. Neither male gender (72% vs. 70.1%, P = 0.87) nor BMI (27.5 vs. 28.0, P = 0.16) was significantly different between NEO and SGX groups.
Compared to patients who received NEO, the SGX group had a significantly lower frequency of non-ischaemic cardiomyopathy as the indication for heart transplantation (44.1% vs. 62.7%, P = 0.021). On the other hand, the NEO group had a lower frequency of heart transplant secondary to ischaemic cardiomyopathy (33.9% vs. 16.4%, P = 0.011). The SGX group had less transplant surgery as the subsequent type of surgery (0.8% vs. 10.4%, P = 0.004), and underwent less thoracic surgery (12.7% vs. 25.4%, P = 0.042) compared with the NEO group.
The median time from transplant day to surgery in years (min-max) was 4.6 (0.0 to 26.1) for the NEO group and 4.4 (0.0 to 25.7) for the SGX group. Both groups mostly comprised patients in ASA-PS class 3 (70.1% vs. 72.9), and pre-operative median LVEF was similar (63%) in both groups as well. Abdominal surgery was the most common surgery for the NEO group (31.5%) as well as for the SGX group (44.9%), P = 0.086 (Table 1).
TABLE 1
Comparisons of characteristics between neostigmine and sugammadex groups
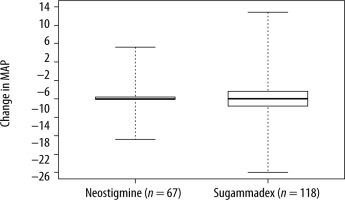
Comparisons of patient outcome variables between NEO and SGX patients are shown in Table 2. In unadjusted analysis, there were no notable differences between the two groups for change in heart rate [0 (–26, 14) vs. 1 (–19, 10), P = 0.59], change in MAP [0 (–22, 28) vs. 0 (–40, 47), P = 0.96], hLOS [2 days (1, 72) vs. 2 (0, 161), P = 0.92] for NEO and SGX respectively, or for intra-operative hypotension [4 (6.0%) vs. 5 (4.2%), OR = 0.70, P = 0.60]. In multivariable analysis (for change in heart rate, change in MAP, and hLOS) adjusting for all the aforementioned baseline/surgical characteristics that differed between the two groups with a P-value < 0.2, the results were similar for change in heart rate (P = 0.59, Figure 2) and change in MAP (P = 0.90, Figure 3). However, there was a significant difference in hLOS between the two groups (P = 0.035), where mean length of stay for the SGX group was 1.53 times that of the NEO group (P = 0.035).
TABLE 2
Comparisons of outcomes between neostigmine and sugammadex groups
[ii] Regression coefficients and 95% CIs are interpreted as the mean difference (sugammadex minus neostigmine) in the given outcome between NEO and SGX groups. The multiplicative effect on the mean and 95% CIs is interpreted as the multiplicative increase in mean length of stay for the sugammadex group compared to the neostigmine group. For hypotension, the odds ratio and 95% CIs are interpreted as the multiplicative increase in the odds of intra-operative hypotension for the sugammadex group compared to the neostigmine group. Multivariable analysis was not performed for intra-operative hypotension outcome given its rare nature.
DISCUSSION
The current study did not reveal significant diffe-rences in intraoperative and postoperative outcomes when comparing patients with a history of heart transplant undergoing subsequent non-cardiac surgery receiving either NEO or SGX. The primary outcomes included the presence of bradycardia and hypotension, which did not show clinically significant variation in any of the studied groups. Similarly, the secondary outcomes, including the need for intraoperative inotropic agents, presence of arrhythmias, cardiac arrest, unplanned ICU admission, and death within 30 days after surgery, were not significantly different in the NEO group compared with the SGX group.
Previous efforts have investigated the rapid effect of NEO versus SGX during neuromuscular blockade recovery, and failed to demonstrate a significant difference in length of hospital stay in non-heart-transplanted patients undergoing non-thoracic procedures comparing both reversal agents [10, 11]. Our study showed a significant difference in hLOS between the two groups, where mean length of stay for the SGX group was 1.53 times that of the NEO. These findings were unexpected and probably explained by clinical features that are incompletely captured during a retrospective review that compelled the clinical team to use SGX over NEO because the patients were deemed at higher risk for unclear reasons.
Initially, it was thought that given the denervation of donors’ hearts, there was minimal or no response to anticholinergic drugs [5, 12]. However, multiple cases of significant bradycardia and cardiac arrest after the administration of these drugs are reported in the literature [6, 13, 14]. Cardiac arrest cases occurred in patients with existing coronary disease as well as a history of rejection, supporting the idea that other patient factors play a significant role [15]. Additionally, there are theories of possible partial denervation, which make the response to these drugs variable and unpredictable [16].
There is also evidence that transplanted hearts gradually gain parasympathetic re-innervation, suggesting that over time patients will respond to a muscarinic stimulus [17]. However, this process is slow and can take up to 15 years to be complete [18]. In our study, the median time from the day of heart transplant to the day of surgery was 4.6 years for NEO and 4.4 years for SGX. This period could also partially explain why there were no significant haemodynamic changes at the time of administration of reversal agents, suggesting that the short period from transplantation to subsequent surgery may not have been long enough for a complete re-innervation.
Backman et al. [6, 19] compared the effect of NEO in patients without heart disease, recent heart transplantation (6 months), and remote heart transplantation (> 6 months). They found a reduction of heart rate in all groups, being more significant in patients without heart transplantation, followed by remote transplant, and lastly, recent transplant. These data support the concept that, over time, the heart will regain some innervation, and the response to direct sympathetic and parasympathetic drugs may occur. These findings align with our study, in which most of our patients underwent remote surgery from the date of heart transplant without experiencing significant intraoperative haemodynamic changes. However, based on multiple cases of bradycardia and cardiac arrest after NEO administration, it may be preferable to avoid NEO in this population.
SGX provides antagonism independent of cholinergic pathways and should not have a significant effect on heart rate, and, therefore, blood pressure. Thus, it may have a role as a good alternative in heart-transplanted patients for NMB reversal. On the other hand, one of the potential side effects of this medication is bradycardia or, rarely, asystole or cardiac arrest [20]. Based on published data, cardiac arrest was mostly associated with anaphylaxis and not due to a direct effect of the drug [21, 22]. In the general population, the evidence showed that the incidence of bradycardia is lower when SGX is used compared with NEO [23]. Although the safety of SGX in patients with heart transplantation was previously demonstrated, some cases of cardiac arrest after its administration have been described [1, 8]. Dahl et al. [24] evaluated the safety of SGX in patients with cardiovascular disease and did not observe any adverse effects. Contradictory evidence showed a lower heart rate and blood pressure in the SGX group compared with NEO [25]. Some studies suggested a possible effect of SGX in reducing catecholamine levels by encapsulation, causing bradycardia and hypotension, especially when high doses are given (16 mg kg-1) [15]. In our study, changes in heart rate and blood pressure were not significantly different after SGX administration in comparison to NEO. In addition, no cases of cardiac arrest occurred in any patient.
Our study is subject to a number of limitations. First, a retrospective study has a higher probability of recollection or entry bias. Second, our sample size (n = 185) is small; however, to our knowledge, this is the largest cohort comparing NEO versus SGX in patients who underwent non-cardiac surgery after receiving a heart transplant. Finally, there are seve-ral factors that can impact haemodynamics during emergence from anaesthesia, and focusing solely on the reversal agent of choice does not portray the entire clinical picture. We did not find any significant haemodynamic change after the administration of NEO or SGX. This could be for multiples reasons, including the routine co-administration of anti-muscarinic agents such as glycopyrrolate or atropine, which could mask bradycardia and/or hypotension.
CONCLUSIONS
There were no notable haemodynamic differences between heart transplant recipient patients undergoing non-cardiac surgery receiving NEO or SGX. The hLOS was longer in the SGX group compared with NEO, but this result was unexpected and not enough data were found to totally explain this finding. We consider that NEO and SGX could be reliably used to reverse neuromuscular blockade during non-cardiac surgery in patients with a prior history of heart transplant. Because of the higher survival rate of heart transplanted patients, the number of these patients undergoing non-cardiac surgery who will need reversal agents is constantly increasing. Therefore, larger studies are needed to better understand the effects of these agents and determine their safety profile.