Introduction
Surgery is commonly employed as a main therapeutic modality for people who have been diagnosed with solid organ malignancies. It is estimated that about eighty percent of cancer patients will require surgical intervention as a crucial element of their treatment protocol [1, 2]. Despite the notable progress in surgical techniques and the incorporation of neoadjuvant and adjuvant medications alongside surgery, a substantial proportion of patients still encounter cancer relapses subsequent to undergoing surgical intervention and curative treatment [3]. The perioperative phase has been acknowledged as a possible determinant of cancer recurrence and long-term survival rates among cancer patients. The incidence of cancer recurrence subsequent to surgical intervention is impacted by a variety of factors, including the primary organ affected by cancer, the stage of tumor node metastasis, and the precise surgical technique utilized [4, 5].
The surgical procedure elicits a multifaceted inflammatory reaction that encompasses the activation of both the innate and adaptive immune systems. This phenomenon enhances the likelihood of localized metastasis and the establishment of novel metastatic sites upon the dissemination of tumor cells into the circulatory system. In addition, the considerable physiological stress and tissue damage caused by surgical interventions trigger activation of the hypothalamic-pituitary-adrenal axis, leading to the release of adrenaline, cortisol, and proinflammatory substances [6]. According to existing research, perioperative interventions, such as the administration of anesthesia and specific anesthetic procedures, have been found to potentially impact the future inflammatory response that occurs after surgery. Additionally, these interventions may possibly have an effect on the probability of cancer recurrence [7]. The impact of anesthetics on cancer recurrence has garnered growing interest in recent years, leading to an upsurge in retrospective studies and preclinical inquiries in this domain. Recent research [8–10] has indicated that the selection of anesthetic procedures for cancer surgery, specifically comparing propofol-based total intravenous anesthesia (TIVA) with volatile anesthesia (VA), may have a significant impact within this field.
Prior research [11, 12] has established that inhalation anesthetics have the capacity to enhance the mechanisms of cell proliferation, migration, invasion, and angiogenesis in several types of cancer cells. In contrast, propofol has been shown to mitigate the aforementioned mechanisms. Multiple in vitro studies have presented evidence suggesting a correlation between the exposure of cancerous cells to volatile anesthetics and increased expression of factors that promote metastasis and tumor growth. Additionally, volatile anesthetics have been found to suppress both innate and adaptive immunity, specifically impacting the activity of natural killer cells. Conversely, propofol has been shown to prevent the upregulation of hypoxia-inducible factor-1α induced by isoflurane and preserve the functionality of the host’s immune system. Research studies have indicated the potential benefits of propofol-based TIVA over volatile anesthesia in terms of the overall survival of patients undergoing cancer surgery. These studies have also provided evidence suggesting a link between the use of inhalational anesthesia and a reduction in recurrence-free survival among cancer patients undergoing elective surgery [13, 14].
However, it is important to note that certain retrospective investigations have produced inconclusive findings on this matter [15]. Therefore, additional evidence is required to reach a definitive conclusion. This systematic review and meta-analysis used up-to-date research on the effects of propofol-based TIVA and volatile anesthesia on long-term oncological outcomes to give strong evidence for clinical practice. The analysis involved the examination of recent findings [16–25] obtained from various databases, including PubMed, Embase, Scopus, and Cochrane.
Aim
The aim of this study was to conduct a systematic review and meta-analysis to examine the comparative effects of TIVA and VA on cancer patients undergoing surgery. Additionally, the study sought to investigate any potential correlation between the administration of both anesthetics and the long-term surgical outcomes of the patients.
Material and methods
Search strategy
The present meta-analysis with the registration number WH#/IRB/2023/1064 was conducted following a comprehensive search across various databases, including PubMed, Embase, Scopus, Cochrane library, and Web of Science. The search covered the period from the year 2000 to 2023 and utilized keywords such as “neoplasm”, “tumor”, “cancer”, “malignancy”, “malignant neoplasm”, “anaesthesia”, “inhalation anesthetics”, “volatile anesthetics”, “total intravenous anesthesia”, “TIVA”, “anesthetic gases”, “balanced anesthesia”, “mortality”, “all-cause mortality”, “survival”, “overall survival”, “recurrence”, “recurrence free survival”, “systematic review”, and “meta-analysis”. Based on the PICOS framework [26], the keywords were identified and found to be consistent in both the Medline and EMBASE databases, as indicated in Table I.
Table I
Database search strategy
| Database | Search strategy |
|---|---|
| Scopus | #1“Neoplasm” OR “tumor” OR “cancer” OR “malignancy” OR “Malignant neoplasm” “Anaesthesia” OR “inhalation anesthetics” OR “volatile anesthetics” OR “anesthetic gases” OR “balanced anesthesia” OR “total intravenous anesthesia” OR “TIVA”, #2 “Mortality,” OR “all-cause mortality,” OR “survival,” OR “overall survival,” OR “recurrence,” OR “recurrence-free survival” #3 #1 AND #2 |
| PubMed | #1 “Neoplasm” OR “tumor” OR “cancer” [MeSH Terms]# OR “malignancy” OR “Malignant neoplasm” [All Fields] OR “volatile anesthetics” OR “anesthetic gases” OR “balanced anesthesia” [All Fields]” OR “Anaesthesia” OR “inhalation anesthetics” [All Fields] OR “total intravenous anesthesia” OR “TIVA” [All Fields] #2 “Mortality,” OR “all-cause mortality,” [MeSH Terms] OR “survival,” OR “overall survival,” OR “recurrence,” OR “recurrence free survival” [All Fields] #3 #1 AND #2 |
| Embase | #1 “Neoplasm” / exp$ OR “tumor”/ exp OR “cancer”/exp OR “malignancy”/exp OR “Malignant neoplasm”/ exp OR “volatile anesthetics”/ exp OR “anesthetic gases” /exp OR “balanced anesthesia”/ exp OR “Anaesthesia” / exp OR “inhalation anesthetics”/exp OR “total intravenous anesthesia”/exp OR “TIVA”/exp #2 “Mortality” / exp OR “all-cause mortality”/ exp OR “Survival”/ exp OR “overall survival” /exp OR “recurrence” OR “recurrence free survival” exp #3 #1 AND #2 |
| Cochrane library | #1 (Neoplasm): ti, ab, kw@ OR (tumor): ti, ab, kw OR (cancer): ti, ab, kw OR (malignancy): ti, ab, kw OR (malignant neoplasm): ti, ab, kw OR (volatile anesthetics): ti, ab, kw OR (anesthetic gases): ti, ab, kw OR (balanced anesthesia): ti, ab, kw OR (inhalation anesthetics):OR (total intravenous anesthesia): ti, ab, kw OR (TIVA) ti, ab, kw (Word variations have been searched) #2 (Mortality): ti, ab, kw OR (all-cause mortality): ti, ab, kw OR (survival): ti, ab, kw or (overall survival): ti, ab, kw or (recurrence): ti, ab, kw or (recurrence free survival): ti, ab, kw (Word variations have been searched) #3 #1 AND #2 |
In the context of searching Scopus, the Title (ti)-Abstract (abs)-keyword (key) field was utilized with the aforementioned keywords. The key phrases “oncological outcome” and “anaesthesia” were utilized in the Cochrane database. The PICO format was utilized to construct precise selection criteria. In this context, “P” denoted cancer patients having surgery, “I” referred to total intravenous anesthesia (TIVA), “C” denoted standard volatile anaesthesia, and “O” encompassed overall survival rate, recurrence-free survival and pathological findings. The inclusion criteria specified that only papers published in the English language were considered. The inclusion of articles was conducted in accordance with the principles outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27]. Two researchers, identified as QY and HL, independently conducted a comprehensive review of the pertinent literature to identify relevant studies.
Inclusion and exclusion criteria
The present analysis included studies that provided information on the comparative utilization of TIVA and VA in the context of surgical interventions for patients with cancer. The selection of studies encompassed the time period from 2000 to 2023. We selected studies that had a full text and provided adequate data for a 2 X 2 table [28]. This meta-analysis incorporated various clinical outcomes as primary measures, including the overall survival rate, recurrence-free survival and pathological findings.
Furthermore, potential correlations between the administration of both anesthetics and the long-term surgical outcomes of the patients were also evaluated. References that were outdated, anecdotal, based on expert opinions, experimental data from animal studies or trials, and studies whose primary data and important information from authors could not be obtained were omitted. Additionally, studies that included surgical patients but not diagnosed with cancer or any other types of malignancies, and papers published in languages other than English were also excluded. The researchers (HY and XY) separately gathered the demographic profile of the patients and event data with significant factors from the included studies.
Quality assessment of included studies
A pre-established standardized questionnaire assessed possible bias in the papers analyzed. The risk of bias was assessed using a Cochrane Collaboration tool [29] that was published in the Cochrane Handbook (version 5.3). The tool included seven items: generating random sequences, concealing allocations, blinding personnel and participants, blinding outcome assessors, selective reporting, incomplete outcome data, and other biases. The assessment of potential bias was conducted separately by two reviewers, QY and HL. A third reviewer, identified as XY, served as an arbitrator to resolve any remaining conflicts. Ultimately, the possible bias was assessed and categorized as either “high risk,” “low risk,” or “unclear risk.” The presence of publication bias was assessed using a funnel plot [30] and Begg’s test [31] was performed using the MedCalc software [32] to determine its statistical significance.
Statistical analysis
The software program Review Manager (RevMan) 5.3 [33] was utilized to assess and analyze the impact of several dichotomous and continuous outcomes. The utilization of reference management software facilitated the organization, extraction, and removal of duplicate references. Forest plots [34] were developed in order to assess the impact of outcome factors across all the investigations. The hazard ratio (HR) [35] was computed using the DerSimonian Lair method, utilizing a 2 X 2 table constructed with event data. The evaluation of dichotomous outcomes involved the use of the HR along with a 95% confidence interval (CI). Heterogeneity was assessed using statistical methods, including the τ2, χ2 test with a matching p-value and the I2 test [36]. If there was heterogeneity between studies, as indicated by an I2 value greater than 50% or a p-value less than 0.05, a random-effects model was employed. Otherwise, a fixed-effect model was used for the pooled analysis [37]. A p-value below 0.05 was deemed to be statistically significant [38]. To evaluate the correlation between administration of TIVA and VA and their long-term post-surgical oncological outcomes in patients, comparative box and whisker plots [39] and scatter plots [40] were constructed.
Results
Literature search results
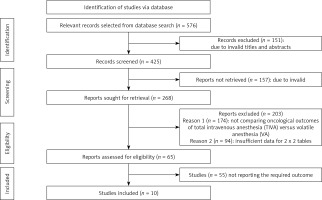
A comprehensive search of several databases was conducted using electronic scanning techniques, resulting in finding a total of 576 studies that met the inclusion criteria outlined by the PICOS framework. A total of 151 papers were removed based on a thorough examination of their titles and abstracts, leaving us with 425 records that underwent further screening. Moreover, as a result of inadequate references and duplications, a total of 157 studies were eliminated from consideration, leaving us with a final pool of 268 papers for further screening. A total of 268 studies were initially considered for inclusion in the analysis. However, after applying the inclusion-exclusion criteria, a total of 203 studies were deemed ineligible and thus eliminated. The remaining 65 papers underwent further assessment to determine their eligibility for inclusion in the analysis. The primary factors contributing to the removal of studies were the absence of a comparison between TIVA and VA for patients who had undergone cancer surgery, insufficient data to construct 2x2 tables, and the unavailability of necessary outcome measures. In this meta-analysis, a total of 10 papers meeting the specified inclusion criteria that span the years from 2016 to 2023 were utilized, as seen in Figure 1.
The studies included in this analysis encompass a total of 14,036 patients who underwent cancer surgery across various age cohorts. The selection of patients for this study was conducted using a random sampling method, and they were thereafter assigned to receive either TIVA or VA intervention prior to undergoing gastrectomy. Table II displays the demographic characteristics of the studies used in this meta-analysis. The text provides a description of the publication’s journal, year of publication, country of study, total number of participants, number of participants in TIVA and VA groups, type of surgery, anesthetics used, age of patients in years, gender (male/female) and reported outcomes. Subsequently, the aforementioned event data were utilized for the purpose of conducting the meta-analysis.
Table II
Brief summary of included studies
| Study ID | Publication year | Journal of publication | Country | Total number of participants | Number of participants (TIVA/VA group) | Surgery | Anesthetics | Age of patients [years] | Gender (M/F) | Reported outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Alexa et al. [16] | 2022 | Trials | Romania | 400 | 300/100 | Colorectal cancer | Volatile anesthesia (VA): sevoflurane or isoflurane or total IV anesthesia (TIVA): propofol | 18–80 | 250/150 | Recurrence-free survival, overall survival, pathological findings |
| Che et al. [17] | 2023 | Frontiers in Surgery | China | 336 | 119/217 | Lung, breast, or esophageal cance | VA: sevoflurane or isoflurane or TIVA: propofol | 55 ±1.5 | 177/159 | Recurrence-free survival, overall survival, pathological findings |
| Dong et al. [18] | 2020 | Journal of Neurosurgical Anesthesiology | China | 294 | 154/140 | Glioma | VA: sevoflurane or isoflurane or TIVA: propofol | ≥ 18 years | 184/110 | Recurrence-free survival, overall survival, pathological findings |
| Hong et al. [19] | 2019 | BMC Anesthesiology | Korea | 2207 | 903/1304 | Gastric, lung, liver, colon, and breast cancer | VA: sevoflurane or isoflurane or TIVA: propofol | 58 ±9 | 1069/1138 | Recurrence-free survival, overall survival, pathological findings |
| Jun et al. [20] | 2017 | Nature Scientific Reports | Korea | 922 | 731/191 | Esophageal cancer surgery | VA: sevoflurane or isoflurane or TIVA: propofol | 63.1 ±7.2 | 866/56 | Recurrence-free survival, overall survival, pathological findings |
| Karami et al. [21] | 2022 | European Journal of Medical Research | Iran | 994 | 530/464 | Breast cancer | VA: sevoflurane or isoflurane or TIVA: propofol | 49.15 ±12.07 | 994 | Recurrence-free survival, overall survival, pathological findings |
| Koo et al. [22] | 2020 | Medical Principles and Practice | South Korea | 259 | 121/138 | Hepatocellular carcinoma | VA: sevoflurane or isoflurane or TIVA : propofol | 61.4±10.3 | 200/59 | Recurrence-free survival, overall survival, pathological findings |
| Oh et al. [23] | 2019 | Acta Anaesthesiologica Scandinavica | Korea | 1538 | 769/769 | Gastric cancer surgery | VA: sevoflurane or isoflurane or TIVA: propofol | ≥ 18 years | 923/615 | Recurrence-free survival, overall survival, pathological findings |
| Sun et al. [24] | 2022 | Medicine (Baltimore) | Taiwan | 56 | 30/26 | Osteosarcoma | VA: sevoflurane or isoflurane or TIVA: propofol | 53 ±12.5 | 33/23 | Recurrence-free survival, overall survival, pathological findings |
| Wigmore et al. [25] | 2016 | Anesthesiology | U.K. | 7030 | 2607/2607 | Gastric, lung, liver, colon, and breast cancer surgery | VA: sevoflurane or isoflurane or TIVA: propofol | 57 ±15.2 | 2596/4434 | Recurrence-free survival, overall survival, pathological findings |
Quality assessment
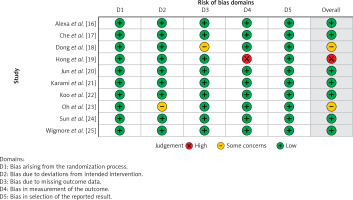
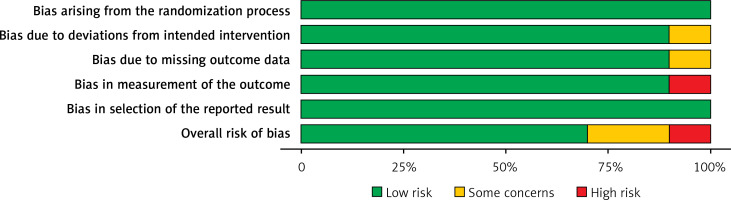
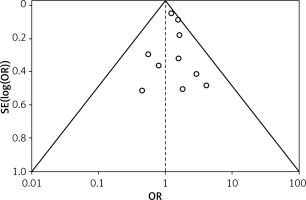
Table III displays the risk of bias assessment results for the included studies, based on the predetermined standardized criteria. The current meta-analysis has a minimal risk of bias, as evidenced by the risk of bias summary (Figure 2) and the risk of bias graph (Figure 3). Out of the 10 studies that were included in the analysis, 7 had a low risk of bias, while 2 were found to have a moderate risk of bias. The moderate risk was attributed to deviations from the intended intervention and missing outcome data. However, one particular study exhibited a significant risk of bias as a result of measuring issues pertaining to the outcome. Additionally, there is a minimal presence of publication bias, indicated from the symmetrical form of the funnel plot depicted in Figure 4, as well as the statistically insignificant p-value of Begg’s test (0.323, which is greater than the predetermined significance level of 0.05).
Table III
Risk assessment of included studies
| Study ID and Year | Alexa et al. [16] | Che et al. [17] | Dong et al. [18] | Hong et al. [19] | Jun et al. [20] | Karami et al. [21] | Koo et al. [22] | Oh et al. [23] | Sun et al. [24] | Wigmore et al. [25] |
|---|---|---|---|---|---|---|---|---|---|---|
| Did the study avoid inappropriate exclusions? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Did all patients receive the same reference standard? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were all patients included in the analysis? | N | N | N | N | N | N | N | N | N | N |
| Was the sample frame appropriate to address the target population? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were study participants sampled in an appropriate way? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the study subjects and the setting described in detail? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were valid methods used for the identification of the condition? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the condition measured in a standard, reliable way for all participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Findings from the statistical analysis
The present meta-analysis consisted of a sample of 10 research articles, involving a total of 14,036 cancer patients. From the total population, 6,264 individuals were given TIVA, whereas 7,772 persons were given standard VA. The statistical analysis was performed on the key outcomes of the study, yielding the subsequent findings.
Overall survival rate of patients in TIVA vs. VA group
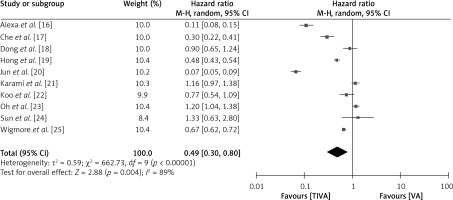
To investigate the comparative impact of administrating TIVA versus VA in cancer patients, the HR for overall survival rate of patients in both groups was examined, as depicted in Figure 5. The TIVA group had a significantly better overall survival rate with a HR of 0.49 (95% CI: 0.30–0.80), a τ2 value of 0.59, χ2 662.73, df 9, Z for overall effect 2.88, I2 89% and p = 0.004.
Recurrence-free survival rate of patients in TIVA vs. VA group
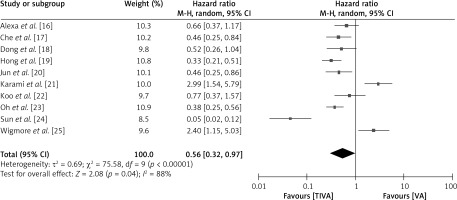
To investigate the comparative impact of administrating TIVA versus VA in cancer patients, the HR for recurrence-free survival rate of patients in both groups was examined, as depicted in Figure 6. The TIVA group had a significantly better recurrence-free survival rate with a hazard HR of 0.56 (95% CI: 0.32–0.97), a τ2 value of 0.69, χ2 75.58, df 9, Z for overall effect 2.08, I2 88% and p = 0.04.
Post surgical pathological findings of patients in TIVA vs. VA group
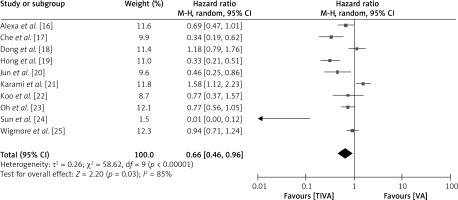
To evaluate the relative impact of administrating TIVA versus VA in cancer patients, the postoperative pathological findings, including postoperative infections, postoperative complications, and postoperative systemic inflammation, were examined, as depicted in Figure 7. The TIVA group had a lower likelihood of post-operative infections with a HR of 0.66 (95% CI: 0.46–0.96), a τ2 value of 0.26, χ2 value of 58.62, df 9, Z for overall effect 2.20, I2 85% and p = 0.03.
Evaluation of potential correlation between administration of TIVA and VA and their long-term surgical outcomes
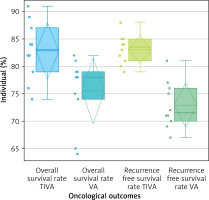
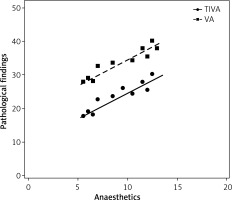
The study examined the correlation between the administration of TIVA and VA and the long-term post-surgical oncological outcomes in patients. This evaluation was conducted using comparative box and whisker plots and scatter plots, as seen in Figures 8 and 9, respectively. The box and whisker plot (Figure 8) provides clear evidence that the administration of TIVA yields substantially improved oncological results compared to the use of VA. The overall survival rate for TIVA exhibited a minimum value of 74, a first quartile (Q1) value of 79, a median value of 83, a third quartile (Q3) value of 87, and a maximum value of 91. The mean (x) was determined to be 82.8, exhibiting a possibly symmetric skewness (p-value of 0.816) and a mesokurtic tail. The values for the VA minima, Q1, median, Q3, and maxima were 64, 74, 78, 79, and 82, respectively. The mean (x) was determined to be 75.5, exhibiting a possibly symmetric skewness (p = 0.082) and a mesokurtic tail. In a similar vein, the recurrence-free survival rate for TIVA exhibited a minimum value of 79, a Q1 value of 81, a median value of 83.5, a Q3 value of 85, and a maximum value of 88. The x was determined to be 83.1, exhibiting a possibly symmetric skewness (p = 0.806) and a mesokurtic tail. The values for the VA minima, Q1, median, Q3, and maxima were 67, 70, 71.5, 76, and 81, respectively. The x was determined to be 72.9, exhibiting a possibly symmetric skewness (p = 0.378) and a mesokurtic tail. The data distribution that correlates with the normal distribution, exhibiting symmetrical data points, a potentially mesokurtic tail, and a limited number of outliers, implies that TIVA offers more effective results in terms of overall survival and recurrence-free survival compared to volatile anesthesia. Similarly, the comparative scatter plot depicted in Figure 9 illustrates the relationship between the administered anesthetics TIVA and VA and the subsequent post-surgical pathological findings. The findings indicate that there is a higher probability of post-surgical complications with VA compared to TIVA. This can be attributed to the greater degree of postoperative immunosuppression associated with VA, which consequently elevates the risk of infection.
Discussion
Cancer is the outcome of the expansion of a clonal group of cells, which occurs via a series of stages known as carcinogenesis. A solitary cell experiences a mutation in essential genes that regulate cell division, cell death, and the preservation of genetic stability, thereby making the cell vulnerable to acquiring other mutations. According to the metastatic cascade, the complex process of cancer metastasis involves numerous tumor-host interactions [41, 42]. Surgical intervention is a primary therapeutic approach employed in the management of individuals diagnosed with solid organ malignancies. Surgical procedures elicit a multifaceted inflammatory response that encompasses the interplay between the innate and adaptive branches of the immune system. There is a prevalent belief that surgical procedures induce a state of postoperative immunosuppression, hence elevating the susceptibility to infections [43, 44]. An increasing corpus of pre-clinical study data suggests that general anesthetic medications have the potential to influence key attributes of cancer that are associated with tumor formation and metastasis [45]. Propofol, etomidate, and ketamine are intravenous sedative-hypnotic drugs often utilized in the context of TIVA induction. The rapid onset of anesthesia is facilitated by their considerable lipophilicity when administered intravenously. Additionally, it facilitates the ability to traverse the blood-brain barrier and achieve efficient perfusion within the cerebral region. TIVA refers to the administration of intravenous medications with the purpose of inducing and sustaining anesthesia. Propofol is the agent that is most commonly utilized. The augmentation of the propofol effect is commonly achieved with the administration of an opioid, such as remifentanil [46–48].
In the field of clinical practice, two main classifications of general anesthetic medications are utilized: intravenous propofol and inhalational volatile anesthetic agents, such as sevoflurane. Volatile anesthetics and intravenous propofol have distinct impacts on both the biology of cancer cells and the immunological response of the host [49, 50]. Despite an increasing number of studies examining the direct impact of anesthetics on the biology of cancer cells, the available data present inconsistent results and are not fully comprehended [51, 52]. Both propofol and volatile anesthetics have unique effects on the immune system and stress response in patients undergoing surgery.
Studies have already shown that volatile anesthetics can make natural killer cells work less well, lower the number of type 1 T helper cells compared to type 2 T helper cells, and cause T lymphocytes to die. In contrast, propofol has the ability to maintain the functionality of natural killer cells. In addition, the use of a volatile anesthetic is linked to a more pronounced stress response compared to propofol-based TIVA [53–55]. As a result, propofol-based TIVA is correlated with improved overall survival. According to data from a retrospective study by Enlund et al. (2014) [56] on 2,838 patients who underwent surgery for colon, rectal, or breast cancer, patients in the propofol group had a 4.7% higher survival rate at 1 year and a 5.5% higher survival rate at 5 years compared to patients receiving volatile inhalational anesthesia. Similar favorable long-term outcomes with propofol-TIVA have also been found in patients undergoing gastrectomy and colectomy in the studies of Zeng et al. (2018) [57] and Wu et al. (2018) [58] respectively. In their article, Wu et al. (2021) [59] discussed the impact of various anesthetic techniques on immune function and oxidative stress in patients undergoing laparoscopic herniorrhaphy. The authors found that, in comparison to intravenous anesthesia (IA), TIVA had a lesser impact on immune function and oxidative stress in these patients. Additionally, TIVA demonstrated more effective control over the inflammatory response and was associated with a lower incidence rate of adverse reactions.
Similarly, in our study, we investigated the effects of TIVA and VA on the long-term oncological outcomes of cancer patients who underwent surgery. Our findings indicated that the TIVA group had a significantly better overall survival rate with a HR of 0.49 (95% CI: 0.30–0.80) and a better recurrence-free survival rate with a HR of 0.56 (95% CI: 0.32–0.97). All observed outcomes were statistically significant (p < 0.05), indicating a preference for the use of TIVA over VA for significant oncological outcomes in patients undergoing cancer surgery.
A significant aspect of this study was the use of comprehensive search terms that cover particular anesthetic drugs and numerous databases. However, it is necessary to illustrate certain limitations. A primary constraint of our study was the omission of research done in languages other than English. Additionally, it is crucial to take into account the potential existence of selection bias in our study, given that a significant number of studies were excluded from our meta-analysis. Furthermore, it remains ambiguous whether the observed outcomes are correlated with variables such as gender, age, and ethnicity. Furthermore, this meta-analysis employed a restricted sample size comprising 10 studies that demonstrated significant variability. Substantial heterogeneity emerged due to variations in management programs, healthcare organizations, type of cancer surgery, duration of surgery, dosages of anesthesia, and follow-up periods among the participants.
Conclusions
The results of the meta-analysis indicate that administration of TIVA provides substantially better oncological outcomes with a higher overall survival rate, a higher recurrence-free survival rate and fewer post-operative pathological findings in patients who underwent surgery for cancer as compared to VA. The findings suggest that TIVA is a more effective anesthetic agent than VA in obtaining better long-term oncological outcomes in cancer patients after surgery. However, it is important to approach the analysis of the results with caution due to the limited number of studies included and the small sample sizes observed in many of these studies. Hence, more research must be undertaken to verify these findings and provide further support for these conclusions.