Diagnosis of allergic diseases is a problem that still cannot be easily overcome. An increasing number of studies have investigated achievement of flow cytometry as a very promising and helpful technique as an allergy diagnostic item. Different types of cells’ identification or activation markers and many research protocols have been shown. However there were no reliable trials that could allow to base the clinical decision on those proposed improvements. Annexin V is a cellular protein that is well known as a marker of early apoptosis but only a few papers mention its possible potential for being a marker of basophil activation [1, 2]. Also, in the previous work from our centre, the diagnostic usefulness of the basophil activation test (BAT) with annexin V in an allergy to Alternaria alternata was demonstrated [3]. To our knowledge, there are still no published papers describing annexin V as a proven basophil activation marker.
The aim of our present, pilot study was to evaluate potential of the new protocol for BAT consist on using CCR3 (C-C chemokine receptor type 3) as an identification marker and annexin V as an activation marker. Endpoints were assessed by measurement of CCR3 expression and changes in annexin V expression on the basophils surface. In order to increase feasibility of the study convenience, sampling methods were used.
The study consisted of 69 participants (29 males; median age: 23 years) being under clinical control in the Department of Internal Medicine, Pneumonology and Allergology, Wroclaw Medical University. The group of 37 patients (18 males; median age: 23 years) was evaluated at admission and met the inclusion criterion: allergy to rye (Secale cereale). The control group of healthy volunteers comprised 32 participants (11 males; median age: 22 years) without any clinical symptoms of allergy (coexistence of any allergies was considered as an exclusion criterion). All participants signed an informed consent form. After enrolment, both study groups were diagnosed with skin prick tests (Allergopharma, Nexter) and allergen-specific IgE immunoglobulin concentrations in the blood was determined by the fluoro-immunoenzymatic method (UniCAP 100 apparatus, Phadia). Afterwards, BAT for bonding of annexin V on the basophil surface was performed with the use of Secale allergen (extract from Allergopharma was diluted in Bühlmann Laboratories solution in three concentrations: C1 = 500 SBU/ml, C2 = 50 SBU/ml, C3 = 5 SBU/ml – Figure 1); it is similar to the methodology of research described by Gonzàles-Muńoz et al. [4] and also accepted by de Weck et al. [5]. The study was performed in 2015–2016 during the period of reduced exposure to allergens (from November to April). The statistical analysis methods were selected based on variables distribution evaluation (Statistica 12.5 with medical pack, StatSoft Polska Sp. z o.o.). The distribution of data was checked by Shapiro-Wilk test. Lack of normally distribution of the data implied use of the U Mann-Whitney test. The diagnostic usefulness of various allergen concentrations in BAT and allergen-specific IgE antibody was assessed by receiver operating characteristic (ROC). Youden index was used for determination of the optimal threshold for which the sum of sensitivity and specificity is maximal [6]. Also, the conjoined specificity and sensitivity of both tests were compared. The procedures in our study were in accordance with the latest version of the Declaration of Helsinki issued by the World Medical Association. The study was approved by the Ethics Committee at the Wroclaw Medical University, Poland.
Figure 1
A – Gating strategy, B – basophil activation without and after allergen stimulation, C – dose dependent allergen induced basophil activation – individual data
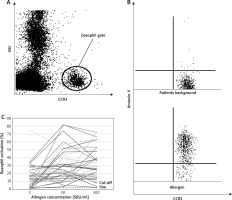
As a result, the significant statistical differences between groups were observed after stimulation with all Secale allergen extracts. No statistical differences were obtained in basophil activation between specific (Pb – patients background) and nonspecific (Pc – positive control) stimulation. In comparison to the reference method (allergen-specific IgE antibody test vs BAT), results obtained in this study showed the same sensitivity (97% vs. 97%) and greater specificity (97% vs. 100%). Moreover, when combining these two methods together (allergen-specific IgE antibody test + BAT), the specificity and sensitivity reached 100%.
The results obtained can lead to greater diagnostic possibilities within the tested allergy profile. Specificity and sensitivity results for annexin V were comparable to results of widely used CD63 which is considered to be one of the standard markers for basophil activation tests [7, 8]. Stability of the anti-CCR3 as an identification marker was confirmed. Advantage of the proposed protocol is that it does not require usage of any additional dyes and its expression does not change significantly after basophil activation. The downside is that it also includes the present activated Th2 lymphocytes. However, Th2 lymphocytes do not constitute a significant fraction of identified cells [9]. Therefore, using annexin V in BAT is justified and worth more detailed research that will confirm its clinical utility. Moreover, in the proposed protocol both preparation of the sample and conduction of the procedure have been simplified. Thanks to simultaneous performance of identification and basophil stimulation, the duration of the procedure has been shortened and the cost of the procedure has been cut by using single markers at the crucial stages of the test (identification and activation) and possible use of allergen extracts used for skin prick tests. The above obtained data on using annexin V as the basophil activation marker may contribute to emergence of the specific and sensitive hi-tech diagnostic method. This method would be especially useful to avoid severe complications during diagnostics and also in patients with some limitation of the standard methods like a suppressed immune system, active skin diseases, pregnancy or use it in children population [10]. Our pilot study has a potentially significant drawback – a relatively small sample size and relies on the improved sensitivity and specificity. Therefore, the study is to be continued in order to achieve a much larger sample. In conclusion, we present our findings to draw attention to the potential role of BAT as a next allergy diagnosis method. With appropriately matched identification, protocols and activation markers, flow cytometry can be a sufficient technique to become the routine diagnosis test.








